LH Levels May Be Used as an Indicator for the Time of Antagonist Administration in GnRH Antagonist Protocols-A Proof-Of-Concept Study
- PMID: 30809195
- PMCID: PMC6379248
- DOI: 10.3389/fendo.2019.00067
LH Levels May Be Used as an Indicator for the Time of Antagonist Administration in GnRH Antagonist Protocols-A Proof-Of-Concept Study
Abstract
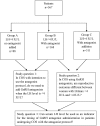
Objective: To investigate whether circulating LH levels could be used as an indicator for the timing of antagonist addition in GnRH antagonist protocol. Design: Retrospective cohort study. Setting: University-based hospital. Patients: A total of 567 women stimulated with recombinant FSH monotherapy in a GnRH antagonist protocol were studied. Among them, 256 patients showed relatively low LH levels [highest LH level (LHmax) < 4 IU/L] during the entire ovarian stimulation process; 88 (Group A) and 168 patients (Group B) were stimulated without and with antagonist co-treatment, respectively. The remaining 311 patients had LHmax≥4 IU/L and were stimulated with a modified flexible antagonist protocol based on LH levels (Group C). Intervention(s): Patients in Group B and C received antagonist during ovarian stimulation, whereas patients in Group A did not. Main outcome measure: Clinical pregnancy rate and ongoing pregnancy rate. Results: The clinical and ongoing pregnancy rates were significantly higher in group A than group B (69.3 vs. 54.7%, P = 0.03 and 62.5 vs. 48.2%, P = 0.04, respectively), but the primary outcome measures did not differ between groups B and C. There were no significant differences in terms of patient demographics, LH levels, total dosage of gonadotrophin, duration of stimulation, follicular output rate between groups A and B, and between groups B and C. Also, there were no significant differences in laboratory and clinical outcomes in pairwise group comparisons. No canceled cycles due to premature ovulation was reported among the treated patients. Conclusion: LH levels may be used as an indicator for the time of antagonist addition. Patients with sustained low LH levels (LHmax<4 IU/L) during controlled ovarian stimulation (COS) might not require antagonist administration. Although further well-designed randomized controlled trials (RCTs) are needed to confirm our results, a novel treatment regimen based on LH measurements during COS might provide clinicians new insights about when to start antagonist administration in the GnRH antagonist protocol.
Keywords: GnRH antagonist; LH level; assisted reproductive technology; controlled ovarian stimulation; pregnancy outcomes.
Similar articles
-
Recombinant follicle-stimulating hormone and recombinant luteinizing hormone versus recombinant follicle-stimulating hormone alone during GnRH antagonist ovarian stimulation in patients aged ≥35 years: a randomized controlled trial.Hum Reprod. 2015 May;30(5):1188-95. doi: 10.1093/humrep/dev038. Epub 2015 Mar 3. Hum Reprod. 2015. PMID: 25740882 Clinical Trial.
-
Recombinant LH supplementation to a standard GnRH antagonist protocol in women of 35 years or older undergoing IVF/ICSI: a randomized controlled multicentre study.Hum Reprod. 2013 Oct;28(10):2804-12. doi: 10.1093/humrep/det266. Epub 2013 Jul 9. Hum Reprod. 2013. PMID: 23838159 Clinical Trial.
-
Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles.Hum Reprod. 2016 Jun;31(6):1253-64. doi: 10.1093/humrep/dew051. Epub 2016 Apr 8. Hum Reprod. 2016. PMID: 27060174 Clinical Trial.
-
The effect of LH supplementation to the GnRH antagonist protocol in advanced reproductive ageing women: a prospective randomized controlled study.Clin Endocrinol (Oxf). 2016 Jan;84(1):99-106. doi: 10.1111/cen.12886. Epub 2015 Sep 9. Clin Endocrinol (Oxf). 2016. PMID: 26304041 Clinical Trial.
-
[Medroxyprogesteron acetate use to block LH surge in oocyte donor stimulation].Ceska Gynekol. 2018 Winter;83(1):11-16. Ceska Gynekol. 2018. PMID: 29510633 Review. Czech.
Cited by
-
Luteinising hormone-based protocol versus traditional flexible gonadotropin-releasing hormone antagonist protocol in women with normal ovarian response: study protocol for a non-inferiority trial.BMJ Open. 2021 Aug 18;11(8):e047974. doi: 10.1136/bmjopen-2020-047974. BMJ Open. 2021. PMID: 34408042 Free PMC article.
-
Response to comment on: Toward an optimal contraception dosing strategy.PLoS Comput Biol. 2024 Dec 18;20(12):e1012649. doi: 10.1371/journal.pcbi.1012649. eCollection 2024 Dec. PLoS Comput Biol. 2024. PMID: 39693379 Free PMC article. No abstract available.
-
Patients With Deep Ovarian Suppression Following GnRH Agonist Long Protocol May Benefit From a Modified GnRH Antagonist Protocol: A Retrospective Cohort Study.Front Endocrinol (Lausanne). 2021 Jul 13;12:618580. doi: 10.3389/fendo.2021.618580. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34326810 Free PMC article.
-
Toward an optimal contraception dosing strategy.PLoS Comput Biol. 2023 Apr 13;19(4):e1010073. doi: 10.1371/journal.pcbi.1010073. eCollection 2023 Apr. PLoS Comput Biol. 2023. PMID: 37053167 Free PMC article.
-
Low Serum LH Levels During Ovarian Stimulation With GnRH Antagonist Protocol Decrease the Live Birth Rate After Fresh Embryo Transfers but Have No Impact in Freeze-All Cycles.Front Endocrinol (Lausanne). 2021 Apr 23;12:640047. doi: 10.3389/fendo.2021.640047. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33967956 Free PMC article.
References
LinkOut - more resources
Full Text Sources