Mitochondria-Responsive Drug Release along with Heat Shock Mediated by Multifunctional Glycolipid Micelles for Precise Cancer Chemo-Phototherapy
- PMID: 30809302
- PMCID: PMC6376467
- DOI: 10.7150/thno.31022
Mitochondria-Responsive Drug Release along with Heat Shock Mediated by Multifunctional Glycolipid Micelles for Precise Cancer Chemo-Phototherapy
Abstract
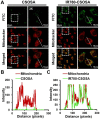
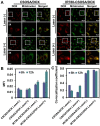
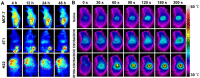
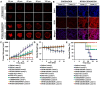
Responsive drug release in tumor mitochondria is a pre-requisite for mitochondria-targeted drug delivery systems to improve the efficacy of this promising therapeutic modality. To this end, a photothermal stimulation strategy for mitochondria-responsive drug release along with heat shock is developed to maximize the antitumor effects with minimal side effects. Methods: This strategy relies on mitochondrial-targeted delivery of doxorubicin (DOX) through a photothermal and lipophilic agent IR-780 iodide (IR780)-modified glycolipid conjugates (CSOSA), which can synergistically triggers high-level reactive oxygen species (ROS) to kill tumor cells. Results: Specifically, upon laser irradiation, the photothermal conversion by IR780-CSOSA can not only weaken the hydrophobic interaction between the core of micelles and DOX and trigger unexpected micelle swelling to release DOX in mitochondria for the amplification of ROS, but also induce mitochondria-specific heat shock to promote the fast evolution of ROS at the same locus to eradicate cancer cells in a more effective way. Furthermore, IR780-CSOSA micelles may independently realize the real-time diagnosis and imaging on multiple tumor models. Deep penetration into tumors by IR780-CSOSA/DOX micelles can be manipulated under laser irradiation. Conclusion: Such multifunctional IR780-CSOSA/DOX micelles with integration of mitochondria-responsive drug release and heat shock are demonstrated to be superior to the non-mitochondria-responsive therapy. This study opens up new avenues for the future cancer diagnosis and treatment.
Keywords: chemotherapy; glycolipid micelles; mitochondria-responsive drug release; mitochondrial heat shock; photothermal therapy.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
Thermo- and pH-dual responsive polymeric micelles with upper critical solution temperature behavior for photoacoustic imaging-guided synergistic chemo-photothermal therapy against subcutaneous and metastatic breast tumors.Theranostics. 2018 Jul 16;8(15):4097-4115. doi: 10.7150/thno.26195. eCollection 2018. Theranostics. 2018. PMID: 30128039 Free PMC article.
-
Mitochondrial alkaline pH-responsive drug release mediated by Celastrol loaded glycolipid-like micelles for cancer therapy.Biomaterials. 2018 Feb;154:169-181. doi: 10.1016/j.biomaterials.2017.07.036. Epub 2017 Jul 31. Biomaterials. 2018. PMID: 29128845
-
A multifunctional nano-delivery system enhances the chemo-co-phototherapy of tumor multidrug resistance via mitochondrial-targeting and inhibiting P-glycoprotein-mediated efflux.J Mater Chem B. 2021 Nov 17;9(44):9174-9182. doi: 10.1039/d1tb01658j. J Mater Chem B. 2021. PMID: 34698329
-
Dual pH/reduction-responsive hybrid polymeric micelles for targeted chemo-photothermal combination therapy.Acta Biomater. 2018 Jul 15;75:371-385. doi: 10.1016/j.actbio.2018.05.026. Epub 2018 May 17. Acta Biomater. 2018. PMID: 29777957
-
Precision Nanomedicines: Targeting Hot Mitochondria in Cancer Cells.ACS Appl Bio Mater. 2022 Sep 6. doi: 10.1021/acsabm.2c00641. Online ahead of print. ACS Appl Bio Mater. 2022. PMID: 36066886 Review.
Cited by
-
Targeted Heating of Mitochondria Greatly Augments Nanoparticle-Mediated Cancer Chemotherapy.Adv Healthc Mater. 2020 Jul;9(14):e2000181. doi: 10.1002/adhm.202000181. Epub 2020 Jun 17. Adv Healthc Mater. 2020. PMID: 32548935 Free PMC article.
-
Multifunctional Mitochondria-Targeting Nanosystems for Enhanced Anticancer Efficacy.Front Bioeng Biotechnol. 2021 Nov 24;9:786621. doi: 10.3389/fbioe.2021.786621. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34900973 Free PMC article. Review.
-
Mitochondria-Targeted and Resveratrol-Loaded Dual-Function Titanium Disulfide Nanosheets for Photothermal-Triggered Tumor Chemotherapy.Nanoscale Res Lett. 2019 Jun 21;14(1):211. doi: 10.1186/s11671-019-3044-5. Nanoscale Res Lett. 2019. PMID: 31227943 Free PMC article.
-
Bubble-Manipulated Local Drug Release from a Smart Thermosensitive Cerasome for Dual-Mode Imaging Guided Tumor Chemo-Photothermal Therapy.Theranostics. 2019 Oct 18;9(26):8138-8154. doi: 10.7150/thno.36762. eCollection 2019. Theranostics. 2019. PMID: 31754386 Free PMC article.
-
GLUT1 targeting and hypoxia-activating polymer-drug conjugate-based micelle for tumor chemo-thermal therapy.Drug Deliv. 2021 Dec;28(1):2256-2267. doi: 10.1080/10717544.2021.1992039. Drug Deliv. 2021. PMID: 34668823 Free PMC article.
References
-
- Tiwari M. Nano cancer therapy strategies. J Cancer Res Ther. 2012;8:19–22. - PubMed
-
- Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng. 2006;8:343–75. - PubMed
-
- Maity AR, Stepensky D. Delivery of drugs to intracellular organelles using drug delivery systems: Analysis of research trends and targeting efficiencies. Int J Pharm. 2015;496:268–74. - PubMed
-
- Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

