Lectin-Mediated pH-Sensitive Doxorubicin Prodrug for Pre-Targeted Chemotherapy of Colorectal Cancer with Enhanced Efficacy and Reduced Side Effects
- PMID: 30809306
- PMCID: PMC6376480
- DOI: 10.7150/thno.29989
Lectin-Mediated pH-Sensitive Doxorubicin Prodrug for Pre-Targeted Chemotherapy of Colorectal Cancer with Enhanced Efficacy and Reduced Side Effects
Abstract
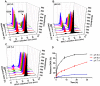
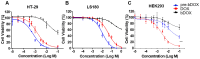
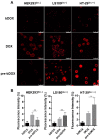
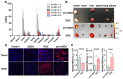
Doxorubicin (DOX) has been clinically used as a broad-spectrum chemotherapeutic agent for decades, but its clinical application is hindered by the lack of tumour specificity, severe cardiotoxicity and haematotoxicity. Pre-targeted strategies are highly tumour-specific, therapeutic approaches. Herein, a novel pre-targeted system was constructed, aiming to enhance anticancer efficacy of DOX and maximally reduce its side effects. Methods: The DOX prodrug (bDOX) was first synthesized by conjugating DOX with mini-PEGylated (mPEGylated) biotin through a pH-sensitive bond. During the pre-targeted treatment, avidin was first administrated. After an optimized interval, bDOX was second administrated. The nontoxic prodrug bDOX was eventually transformed into the toxic anticancer form (DOX) by a pH-triggered cleavage specifically in tumour cells. The drug efficacy and side effect of the two-step, pre-targeted treatment were fully compared with free DOX in vitro and in vivo. Results: The prodrug bDOX was quite stable under neutral conditions and nearly nontoxic, but was immediately transformed into the toxic anticancer form (DOX) under acidic conditions. Compared to free DOX, the pre-targeted bDOX exhibited a higher cellular uptake by human colorectal tumour cells (LS180 and HT-29 cells). In vivo evaluation performed on LS180 xenograft animal model demonstrated that the pre-targeted bDOX achieved a much more significant tumour inhibition than free DOX. The largely decreased, unwanted bystander toxicity was demonstrated by changes in body weight, cardiomyocyte apoptosis, blood routine examination and splenic pathological changes. Conclusion: The high therapeutic efficacy, together with the minimal side effects, of this easily synthesized, pre-targeted system exhibited immense potentiality for the clinical application of DOX delivery.
Keywords: avidin; biotin; chemotherapy; doxorubicin prodrug; pre-targeted strategy.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Jehn CF, Hemmati P, Lehenbauer-Dehm S, Kummel S, Flath B, Schmid P. Biweekly pegylated liposomal doxorubicin (Caelyx) in heavily pretreated metastatic breast cancer: a phase 2 study. Clin Breast Cancer. 2016;16:514–9. - PubMed
-
- Damiani RM, Moura DJ, Viau CM, Caceres RA, Henriques JAP, Saffi J. Pathways of cardiac toxicity: comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch Toxicol. 2016;90:2063–76. - PubMed
-
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. - PubMed
-
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. - PubMed
-
- Bildstein L, Dubernet C, Couvreur P. Prodrug-based intracellular delivery of anticancer agents. Adv Drug Deliv Rev. 2011;63:3–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

