Targeted Drug Delivery and Image-Guided Therapy of Heterogeneous Ovarian Cancer Using HER2-Targeted Theranostic Nanoparticles
- PMID: 30809308
- PMCID: PMC6376473
- DOI: 10.7150/thno.29964
Targeted Drug Delivery and Image-Guided Therapy of Heterogeneous Ovarian Cancer Using HER2-Targeted Theranostic Nanoparticles
Abstract
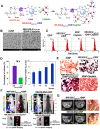
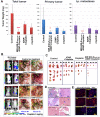
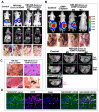
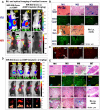
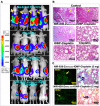
Cancer heterogeneity and drug resistance limit the efficacy of cancer therapy. To address this issue, we have developed an integrated treatment protocol for effective treatment of heterogeneous ovarian cancer. Methods: An amphiphilic polymer coated magnetic iron oxide nanoparticle was conjugated with near infrared dye labeled HER2 affibody and chemotherapy drug cisplatin. The effects of the theranostic nanoparticle on targeted drug delivery, therapeutic efficacy, non-invasive magnetic resonance image (MRI)-guided therapy, and optical imaging detection of therapy resistant tumors were examined in an orthotopic human ovarian cancer xenograft model with highly heterogeneous levels of HER2 expression. Results: We found that systemic delivery of HER2-targeted magnetic iron oxide nanoparticles carrying cisplatin significantly inhibited the growth of primary tumor and peritoneal and lung metastases in the ovarian cancer xenograft model in nude mice. Differential delivery of theranostic nanoparticles into individual tumors with heterogeneous levels of HER2 expression and various responses to therapy were detectable by MRI. We further found a stronger therapeutic response in metastatic tumors compared to primary tumors, likely due to a higher level of HER2 expression and a larger number of proliferating cells in metastatic tumor cells. Relatively long-time retention of iron oxide nanoparticles in tumor tissues allowed interrogating the relationship between nanoparticle drug delivery and the presence of resistant residual tumors by in vivo molecular imaging and histological analysis of the tumor tissues. Following therapy, most of the remaining tumors were small, primary tumors that had low levels of HER2 expression and nanoparticle drug accumulation, thereby explaining their lack of therapeutic response. However, a few residual tumors had HER2-expressing tumor cells and detectable nanoparticle drug delivery but failed to respond, suggesting additional intrinsic resistant mechanisms. Nanoparticle retention in the small residual tumors, nevertheless, produced optical signals for detection by spectroscopic imaging. Conclusion: The inability to completely excise peritoneal metastatic tumors by debulking surgery as well as resistance to chemotherapy are the major clinical challenges for ovarian cancer treatment. This targeted cancer therapy has the potential for the development of effective treatment for metastatic ovarian cancer.
Keywords: MR image-guided cancer therapy; resistant mechanism; spectroscopic imaging; targeted drug delivery; theranostic nanoparticles.
Conflict of interest statement
Competing interests: Dr. Y. Andrew Wang is the President and Principal Scientist at Ocean Nanotech LLC, San Diego, CA. All other authors have declared that no conflict of interest exists.
Figures


References
-
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–87. - PubMed
-
- Talavera A, Friemann R, Gomez-Puerta S, Martinez-Fleites C, Garrido G, Rabasa A. et al. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Cancer Res. 2009;69:5851–9. - PubMed
-
- Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annu Rev Med. 2013;64:15–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

