Nanoparticle Binding to Urokinase Receptor on Cancer Cell Surface Triggers Nanoparticle Disintegration and Cargo Release
- PMID: 30809315
- PMCID: PMC6376475
- DOI: 10.7150/thno.29445
Nanoparticle Binding to Urokinase Receptor on Cancer Cell Surface Triggers Nanoparticle Disintegration and Cargo Release
Abstract
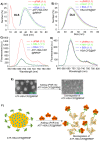
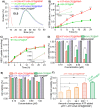
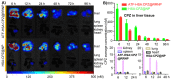
Cancer cell expresses abundant surface receptors. These receptors are important targets for cancer treatment and imaging applications. Our goal here is to develop nanoparticles with cargo loading and tumor targeting capability. Methods: A peptide targeting at cancer cell surface receptor (urokinase receptor, uPAR) was expressed in fusion with albumin (diameter of ~7 nm), and the fusion protein was assembled into nanoparticles with diameter of 40 nm, either in the presence or absence of cargo molecules, by a novel preparation method. An important feature of this method is that the nanoparticles were stabilized by hydrophobic interaction of the fusion protein and no covalent linking agent was used in the preparation. The stability, the cargo release, in vitro and in vivo properties of such formed nanoparticles were characterized by transmission electron microscopy, dynamic light scattering, gel shift assay, laser scanning confocal microscopy and 3D fluorescent molecular tomography. Results: The nanoparticles were stable for more than two weeks in aqueous buffer, even in the buffer containing 10% fetal bovine serum. Interestingly, in the presence of urokinase receptor, the uPAR-targeting nanoparticle disintegrated into 7.5 nm fragments and released its cargo, but not the non-targeting nanoparticles made from albumin by the same preparation method. Such nanoparticles also showed higher uptake and cytotoxicity to the receptor-expressing cancer cells in vitro and higher tumor accumulation in xenografted tumor-bearing mice in vivo compared to the non-targeting nanoparticles. Conclusion: Our results demonstrate a new function of cell surface receptor as a responsive trigger to disassemble nanoparticles, besides its common use to enrich targeting agents. Such nanoparticles were thus named receptor-responsive nanoparticles (RRNP).
Keywords: amino terminal fragment of urokinase-type plasminogen activator; cargo release; human serum albumin; receptor-triggered disintegration; urokinase receptor.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–71. - PubMed
-
- Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24:1211–7. - PubMed
-
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

