Excessive Oxidative Stress Contributes to Increased Acute ER Stress Kidney Injury in Aged Mice
- PMID: 30809321
- PMCID: PMC6369482
- DOI: 10.1155/2019/2746521
Excessive Oxidative Stress Contributes to Increased Acute ER Stress Kidney Injury in Aged Mice
Abstract
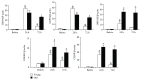
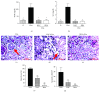
The aged kidney is susceptible to acute injury due presumably to its decreased ability to handle additional challenges, such as endoplasmic reticulum (ER) stress. This was tested by giving tunicamycin, an ER stress inducer, to either old or young mice. Injection of high dose caused renal failure in old mice, not in young mice. Moreover, injection of low dose resulted in severe renal damage in old mice, confirming the increased susceptibility of aged kidney to ER stress. There existed an abnormality in ER stress response kinetics in aged kidney, characterized by a loss of XBP-1 splicing and decreased PERK-eIF2α phosphorylation at late time point. The presence of excessive oxidative stress in aged kidney may play a role since high levels of oxidation increased ER stress-induced cell death and decreased IRE1 levels and XBP-1 splicing. Importantly, treatment with antioxidants protected old mice from kidney injury and normalized IRE1 and XBP-1 responses. Furthermore, older mice (6 months old) transgenic with antioxidative stress AGER1 were protected from ER stress-induced kidney injury. In conclusion, the decreased ability to handle ER stress, partly due to the presence of excessive oxidative stress, may contribute to increased susceptibility of the aging kidney to acute injury.
Figures




Similar articles
-
Increased susceptibility to acute kidney injury due to endoplasmic reticulum stress in mice lacking tumor necrosis factor-α and its receptor 1.Kidney Int. 2011 Mar;79(6):613-623. doi: 10.1038/ki.2010.469. Epub 2010 Dec 8. Kidney Int. 2011. PMID: 21150875
-
Endoplasmic reticulum stress in retinal vascular degeneration: protective role of resveratrol.Invest Ophthalmol Vis Sci. 2012 May 31;53(6):3241-9. doi: 10.1167/iovs.11-8406. Invest Ophthalmol Vis Sci. 2012. PMID: 22491413
-
Gender differences control the susceptibility to ER stress-induced acute kidney injury.Am J Physiol Renal Physiol. 2013 Apr 1;304(7):F875-82. doi: 10.1152/ajprenal.00590.2012. Epub 2013 Jan 30. Am J Physiol Renal Physiol. 2013. PMID: 23364800 Free PMC article.
-
Intrarenal renin-angiotensin system mediates fatty acid-induced ER stress in the kidney.Am J Physiol Renal Physiol. 2016 Mar 1;310(5):F351-63. doi: 10.1152/ajprenal.00223.2015. Epub 2015 Dec 16. Am J Physiol Renal Physiol. 2016. PMID: 26672616 Free PMC article.
-
Tight junction proteins and oxidative stress in heavy metals-induced nephrotoxicity.Biomed Res Int. 2013;2013:730789. doi: 10.1155/2013/730789. Epub 2013 Apr 22. Biomed Res Int. 2013. PMID: 23710457 Free PMC article. Review.
Cited by
-
Renoprotective effect of Limonium duriusculum (de Girard) Kuntze via modulation of oxidative stress/ UPR markers and inflammation during cyclosporine-induced nephrotoxicity in rats.Iran J Basic Med Sci. 2024;27(8):1023-1032. doi: 10.22038/IJBMS.2024.77052.16661. Iran J Basic Med Sci. 2024. PMID: 38911250 Free PMC article.
-
The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling.Exp Mol Med. 2021 Feb;53(2):151-167. doi: 10.1038/s12276-021-00560-8. Epub 2021 Feb 8. Exp Mol Med. 2021. PMID: 33558590 Free PMC article. Review.
-
The Role of the Unfolded Protein Response on Renal Lipogenesis in C57BL/6 Mice.Biomolecules. 2021 Jan 7;11(1):73. doi: 10.3390/biom11010073. Biomolecules. 2021. PMID: 33430288 Free PMC article.
-
The role of extracellular vesicles in podocyte autophagy in kidney disease.J Cell Commun Signal. 2021 Sep;15(3):299-316. doi: 10.1007/s12079-020-00594-z. Epub 2021 Feb 22. J Cell Commun Signal. 2021. PMID: 33619681 Free PMC article. Review.
-
Selective Activation of Endoplasmic Reticulum Stress by Reactive-Oxygen-Species-Mediated Ochratoxin A-Induced Apoptosis in Tubular Epithelial Cells.Int J Mol Sci. 2021 Oct 11;22(20):10951. doi: 10.3390/ijms222010951. Int J Mol Sci. 2021. PMID: 34681610 Free PMC article.
References
-
- Chen G., Bridenbaugh E. A., Akintola A. D., et al. Increased susceptibility of aging kidney to ischemic injury: identification of candidate genes changed during aging, but corrected by caloric restriction. American Journal of Physiology-Renal Physiology. 2007;293(4):F1272–F1281. doi: 10.1152/ajprenal.00138.2007. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

