Catalytic farming: reaction rotation extends catalyst performance
- PMID: 30809358
- PMCID: PMC6354835
- DOI: 10.1039/c8sc04188a
Catalytic farming: reaction rotation extends catalyst performance
Abstract
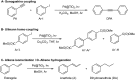
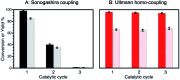
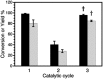
The use of heterogeneous catalysis has key advantages compared to its homogeneous counterpart, such as easy catalyst separation and reusability. However, one of the main challenges is to ensure good performance after the first catalytic cycles. Active catalytic species can be inactivated during the catalytic process leading to reduced catalytic efficiency, and with that loss of the advantages of heterogeneous catalysis. Here we present an innovative approach in order to extend the catalyst lifetime based on the crop rotation system used in agriculture. The catalyst of choice to illustrate this strategy, Pd@TiO2, is used in alternating different catalytic reactions, which reactivate the catalyst surface, thus extending the reusability of the material, and preserving its selectivity and efficiency. As a proof of concept, different organic reactions were selected and catalyzed by the same catalytic material during target molecule rotation.
Figures





Similar articles
-
Metal-organic frameworks: versatile heterogeneous catalysts for efficient catalytic organic transformations.Chem Soc Rev. 2015 Oct 7;44(19):6804-49. doi: 10.1039/c4cs00395k. Chem Soc Rev. 2015. PMID: 25958955
-
Deformable Metal-Organic Framework Nanosheets for Heterogeneous Catalytic Reactions.J Am Chem Soc. 2020 May 20;142(20):9408-9414. doi: 10.1021/jacs.0c02272. Epub 2020 Apr 24. J Am Chem Soc. 2020. PMID: 32302117
-
Multi-Catalytic Metal-Based Homogeneous-Heterogeneous Systems in Organic Chemistry.Chemistry. 2024 Sep 5;30(50):e202400443. doi: 10.1002/chem.202400443. Epub 2024 Aug 20. Chemistry. 2024. PMID: 38958991
-
Potential Utilization of Metal-Organic Frameworks in Heterogeneous Catalysis: A Case Study of Hydrogen-Bond Donating and Single-Site Catalysis.Chem Asian J. 2019 Dec 2;14(23):4087-4102. doi: 10.1002/asia.201900823. Epub 2019 Oct 25. Chem Asian J. 2019. PMID: 31591812 Review.
-
Palladium Catalyst Supported on Zeolite for Cross-coupling Reactions: An Overview of Recent Advances.Top Curr Chem (Cham). 2017 Feb;375(1):2. doi: 10.1007/s41061-016-0084-5. Epub 2016 Dec 7. Top Curr Chem (Cham). 2017. PMID: 27928737 Review.
Cited by
-
MultiShapeC, an algorithm to assess concentration in multi-shape nanoparticle samples: nanosilver, a case study.RSC Adv. 2022 Sep 20;12(41):26550-26555. doi: 10.1039/d2ra04078f. eCollection 2022 Sep 16. RSC Adv. 2022. PMID: 36275155 Free PMC article.
-
Structural transformations of solid electrocatalysts and photocatalysts.Nat Rev Chem. 2021 Apr;5(4):256-276. doi: 10.1038/s41570-021-00255-8. Epub 2021 Feb 18. Nat Rev Chem. 2021. PMID: 37117283 Review.
-
Photocatalytic Semi-Hydrogenation of Alkynes: A Game of Kinetics, Selectivity and Critical Timing.Nanomaterials (Basel). 2023 Aug 22;13(17):2390. doi: 10.3390/nano13172390. Nanomaterials (Basel). 2023. PMID: 37686898 Free PMC article.
-
The Dark Side of Lead-Free Metal Halide Nanocrystals: Substituent-Modulated Photocatalytic Activity in Benzyl Bromide Reduction.ACS Energy Lett. 2023 May 30;8(6):2789-2798. doi: 10.1021/acsenergylett.3c00771. eCollection 2023 Jun 9. ACS Energy Lett. 2023. PMID: 37324538 Free PMC article.
References
-
- Biffis A., Centomo P., Del Zotto A., Zeccal M. Chem. Rev. 2018;118:2249. - PubMed
-
- Liu Q., Xu M. D., Zhao J., Yang Z., Qi C. Z., Zeng M. F., Xia R., Cao X. Z., Wang B. Y. Int. J. Biol. Macromol. 2018;113:1308. - PubMed
- Zeng M. F., Zhang X., Shao L. J., Qi C. Z., Zhang X. M. J. Organomet. Chem. 2012;704:29.
- Chen Z. P., Vorobyeva E., Mitchell S., Fako E., Ortuno M. A., Lopez N., Collins S. M., Midgley P. A., Richard S., Vile G., Perez-Ramirez J. Nat. Nanotechnol. 2018;13:702. - PubMed
-
- Argyle M., Bartholomew C. Catalysts. 2015;5:145.
-
- Akcil A., Veglio F., Ferella F., Okudan M. D., Tuncuk A. J. Waste Manage. 2015;45:420. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

