Genome-wide methylation profiling and copy number analysis in atypical fibroxanthomas and pleomorphic dermal sarcomas indicate a similar molecular phenotype
- PMID: 30809375
- PMCID: PMC6375211
- DOI: 10.1186/s13569-019-0113-6
Genome-wide methylation profiling and copy number analysis in atypical fibroxanthomas and pleomorphic dermal sarcomas indicate a similar molecular phenotype
Abstract
Background: Atypical fibroxanthomas (AFX) and pleomorphic dermal sarcomas (PDS) are lesions of the skin with overlapping histologic features and unspecific molecular traits. PDS behaves aggressive compared to AFX. Thus, a precise delineation, although challenging in some instances, is relevant.
Methods: We examined the value of DNA-methylation profiling and copy number analysis for separating these tumors. DNA-methylation data were generated from 17 AFX and 15 PDS using the Illumina EPIC array. These were compared with DNA-methylation data generated from 196 tumors encompassing potential histologic mimics like cutaneous squamous carcinomas (cSCC; n = 19), basal cell carcinomas (n = 10), melanoma metastases originating from the skin (n = 11), leiomyosarcomas (n = 11), angiosarcomas of the skin and soft tissue (n = 11), malignant peripheral nerve sheath tumors (n = 19), dermatofibrosarcomas protuberans (n = 13), extraskeletal myxoid chondrosarcomas (n = 9), myxoid liposarcomas (n = 14), schwannomas (n = 10), neurofibromas (n = 21), alveolar (n = 19) and embryonal (n = 17) rhabdomyosarcomas as well as undifferentiated pleomorphic sarcomas (n = 12).
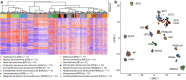
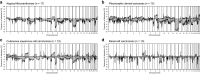
Results: DNA-methylation profiling did not separate AFX from PDS. The DNA-methylation profiles of the other cases, however, were distinct from AFX/PDS. They reliably assigned to subtype-specific DNA-methylation clusters, although overlap occurred between some AFX/PDS and cSCC. Copy number profiling revealed alterations in a similar frequency and distribution between AFX and PDS. They involved losses of 9p (22/32) and 13q (25/32). Gains frequently involved 8q (8/32). Notably, a homozygous deletion of CDKN2A was more frequent in PDS (6/15) than in AFX (2/17), whereas amplifications were non-recurrent and overall rare (5/32).
Conclusions: Our findings support the concept that AFX and PDS belong to a common tumor spectrum. We could demonstrate the diagnostic value of DNA-methylation profiling to delineating AFX/PDS from potential mimics. However, the assessment of certain histologic features remains crucial for separating PDS from AFX.
Keywords: Atypical fibroxanthoma; Carcinomas; DNA methylation; Melanomas; Mimics; Pleomorphic dermal sarcoma; Profiling; Sarcomas.
Figures
References
-
- Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. WHO classification of tumours of soft tissue and bone. Lyon: IARC Press; 2013.
-
- Kraft S, Granter SR. Molecular pathology of skin neoplasms of the head and neck. Arch Pathol Lab Med. 2014;138(6):759–787. - PubMed
-
- Luzar B, Calonje E. Morphological and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37(3):301–309. - PubMed
-
- Mentzel T, Requena L, Brenn T. Atypical fibroxanthoma revisited. Surg Pathol Clin. 2017;10(2):319–335. - PubMed
-
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18(8):50. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

