Longitudinal evolution of CT and MRI LI-RADS v2014 category 1, 2, 3, and 4 observations
- PMID: 30809719
- PMCID: PMC7495398
- DOI: 10.1007/s00330-019-06058-2
Longitudinal evolution of CT and MRI LI-RADS v2014 category 1, 2, 3, and 4 observations
Abstract
Objectives: This study assesses the risk of progression of Liver Imaging Reporting and Data System (LI-RADS) categories, and the effects of inter-exam changes in modality or radiologist on LI-RADS categorization.
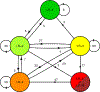
Methods: Clinical LI-RADS v2014 CT and MRI exams at our institution between January 2014 and September 2017 were retrospectively identified. Untreated LR-1, LR-2, LR-3, and LR-4 observations with at least one follow-up exam were included. Three hundred and seventy-two observations in 214 patients (149 male, 65 female, mean age 61 ± 10 years) were included during the study period (715 exams total). Cumulative incidence curves for progression to malignant LI-RADS categories (LR-5 or LR-M) and to LR-4 or higher were generated for each index category and compared using log-rank tests with a resampling extension. Relationships between inter-exam changes in LI-RADS category and modality or radiologist, adjusted for inter-exam time intervals, were modeled using mixed effect logistic regressions.
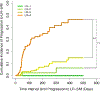
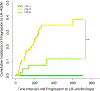
Results: Median inter-exam follow-up interval and total follow-up duration were 123 and 227 days, respectively. Index LR-1, LR-2, LR-3, and LR-4 differed significantly in their cumulative incidences of progression to malignant categories (p < 0.0001), which were 0%, 2%, 7%, and 32% at 6 months, respectively. Index LR-1, LR-2, and LR-3 differed significantly in cumulative incidences of progression to LR-4 or higher (p = 0.003). MRI-MRI exam pairs had more stable LI-RADS categorization compared to CT-CT (OR = 0.460, p = 0.0018).
Conclusions: LI-RADS observations demonstrate increasing risk of progression to malignancy with increasing category ranging from 0% for LR-1 to 32% for LR-4 at 6 months. Inter-exam modality changes are associated with LI-RADS category changes.
Key points: • While the majority of LR-2 observations remain stable over long-term follow-up, LR-3 and especially LR-4 observations have a higher risk for category progression. • Category transitions between sequential exams using different modalities (CT vs. MRI) may reflect modality differences rather than biological change. MRI, especially with the same type of contrast agent, may provide the most reproducible categorization, although this needs additional validation. • In a clinical practice setting, in which radiologists refer to prior imaging and reports, there was no significant association between changes in radiologist and changes in LI-RADS categorization.
Keywords: Hepatic neoplasms; Hepatocellular carcinoma; Liver; Longitudinal studies; Observer variation.
Figures


References
-
- Heimbach JK, Kulik LM, Finn RS, et al. (2018) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67:358–380. - PubMed
-
- Mitchell DG, Bruix J, Sherman M, Sirlin CB (2015) LI-RADS (Liver Imaging Reporting and Data System): Summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 61:1056–1065. - PubMed
-
- Wald C, Russo MW, Heimbach JK, et al. (2013) New OPTN/UNOS Policy for Liver Transplant Allocation: Standardization of Liver Imaging, Diagnosis, Classification, and Reporting of Hepatocellular Carcinoma. Radiology 266:376–382. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

