Conventional transbronchial needle aspiration is promising for identifying EGFR mutations in lung adenocarcinoma
- PMID: 30810282
- PMCID: PMC6449271
- DOI: 10.1111/1759-7714.13014
Conventional transbronchial needle aspiration is promising for identifying EGFR mutations in lung adenocarcinoma
Abstract
Background: Conventional transbronchial needle aspiration (TBNA) is advantageous for the one-step diagnosis and staging of lung adenocarcinoma under topical anesthesia and conscious sedation. We examined its efficacy for identifying EGFR mutations.
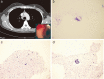
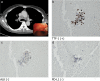
Methods: Forty-seven patients with proven or suspected lung adenocarcinoma indicated for hilar-mediastinal lymph node (LN) staging between June 2011 and December 2017 were enrolled. The cellblock was prepared using the plasma-thrombin method. TaqMan PCR was used to detect mutations. Considering cost effectiveness, only the sample with the highest tumor cell fraction in the same patient was chosen for analysis.
Results: TBNA provided positive results of malignancy in 27 patients. Seventeen patients (63.0%) had cellblocks eligible for mutation testing. Bronchial biopsy (n = 6), neck LN fine needle aspiration (n = 1), and brushing (n = 1), provided higher tumor cell fractions for analysis in eight patients. TBNA was the exclusive method used in nine patients (19.1%). For patients with an inadequate TBNA cellblock, bronchial biopsy (n = 5), neck LN fine needle aspiration (n = 3), computed tomography-guided transthoracic needle biopsy (n = 1), and brushing (n = 1) were used for analysis. Modification to specimen processing to prevent exhaustion by cytology after June 2016 improved the adequacy of cellblock samples (9/10, 90% vs. 8/17, 47.1%; P = 0.042).
Conclusions: These findings suggest the promising role of conventional TBNA and highlight the challenges of doing more with less in an era of precision medicine.
Keywords: Adenocarcinoma; bronchoscopy; epidermal growth factor receptor; lung cancer; transbronchial needle aspiration.
© 2019 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures




References
-
- Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–39. - PubMed
-
- Mok TS, Wu YL, Thongprasert S et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. - PubMed
-
- Gao G, Ren S, Li A et al. Epidermal growth factor receptor‐tyrosine kinase inhibitor therapy is effective as first‐line treatment of advanced non‐small‐cell lung cancer with mutated EGFR: A meta‐analysis from six phase III randomized controlled trials. Int J Cancer 2012; 131: E822–9. - PubMed
-
- Hsu LH, Ko JS, Liu CC. Education and experience improve the performance of transbronchial needle aspiration: A learning curve at a cancer center. Chest 2004; 125: 532–40. - PubMed
-
- Hsu LH, Liu CC, Ko JS, Chen CC, Feng AC. Safety of interventional bronchoscopy through complication review at a cancer center. Clin Respir J 2016; 10: 359–67. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

