Vascular smooth muscle cell contraction and relaxation in the isolated aorta: a critical regulator of large artery compliance
- PMID: 30810292
- PMCID: PMC6391714
- DOI: 10.14814/phy2.13934
Vascular smooth muscle cell contraction and relaxation in the isolated aorta: a critical regulator of large artery compliance
Abstract
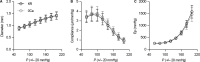
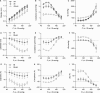
Over the past few decades, isometric contraction studies of isolated thoracic aorta segments have significantly contributed to our overall understanding of the active, contractile properties of aortic vascular smooth muscle cells (VSMCs) and their cross-talk with endothelial cells. However, the physiological role of VSMC contraction or relaxation in the healthy aorta and its contribution to the pulse-smoothening capacity of the aorta is currently unclear. Therefore, we investigated the acute effects of VSMC contraction and relaxation on the isobaric biomechanical properties of healthy mouse aorta. An in-house developed set-up was used to measure isobaric stiffness parameters of periodically stretched (10 Hz) aortic segments at an extended pressure range, while pharmacologically modulating VSMC tone and endothelial cell function. We found that the effects of α1-adrenergic stimulation with phenylephrine on the pressure-stiffness relationship varied in sensitivity, magnitude and direction, with the basal, unstimulated NO production by the endothelium playing a pivotal role. We also investigated how arterial disease affected this system by using the angiotensin-II-treated mouse. Our results show that isobaric stiffness was increased and that the aortic segments demonstrated a reduced capacity for modulating the pressure-stiffness relationship. This suggests that not only increased isobaric stiffness at normal pressure, but also a reduced capacity of the VSMCs to limit the pressure-associated increase in aortic stiffness, may contribute to the pathogenesis of this mouse model. Overall, this study provides more insight in how aortic VSMC tone affects the pressure-dependency of aortic biomechanics at different physiological and pathological conditions.
Keywords: Aortic stiffness; basal NO; mouse; vascular smooth muscle tone.
© 2019 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
None.
Figures



References
-
- Aars, H. 1971. Diameter and elasticity of the ascending aorta during infusion of noradrenaline. Acta Physiol. Scand. 83:133–138. - PubMed
-
- Apter, J. T. , Rabinowitz M., and Cummings D. H.. 1966. Correlation of visco‐elastic properties of large arteries with microscopic structure. Circ. Res. 19:104–121. - PubMed
-
- Armentano, R. , Cabrera E., Levenson J., Barra J., Breitbart G., Pichel R., et al. 1990. Effects of muscarinic and beta‐adrenergic blockade on the aortic elastic response to epinephrine induced acute hypertension in conscious dogs. Am. J. Hypertens. 3:476–481. - PubMed
-
- Bank, A. J. , Wilson R. F., Kubo S. H., Holte J. E., Dresing T. J., and Wang H.. 1995. Direct effects of smooth muscle relaxation and contraction on in vivo human brachial artery elastic properties. Circ. Res. 77:1008–1016. - PubMed
-
- Bank, A. J. , Wang H., Holte J. E., Mullen K., Shammas R., and Kubo S. H.. 1996. Contribution of collagen, elastin, and smooth muscle to in vivo human brachial artery wall stress and elastic modulus. Circulation 94:3263–3270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

