Pharmacokinetics of HLD200, a Delayed-Release and Extended-Release Methylphenidate: Evaluation of Dose Proportionality, Food Effect, Multiple-Dose Modeling, and Comparative Bioavailability with Immediate-Release Methylphenidate in Healthy Adults
- PMID: 30810347
- PMCID: PMC6479242
- DOI: 10.1089/cap.2018.0122
Pharmacokinetics of HLD200, a Delayed-Release and Extended-Release Methylphenidate: Evaluation of Dose Proportionality, Food Effect, Multiple-Dose Modeling, and Comparative Bioavailability with Immediate-Release Methylphenidate in Healthy Adults
Abstract
Objectives: HLD200, an oral, once-daily, evening-dosed, delayed-release, and extended-release methylphenidate (DR/ER-MPH), was designed to provide efficacy from the early morning, throughout the day, and into the evening to individuals with attention-deficit/hyperactivity disorder. The objectives were to evaluate DR/ER-MPH pharmacokinetic (PK) properties in healthy adults, including dose proportionality, food effect, the potential of accumulation using multiple-dose modeling, and bioavailability compared to an immediate-release MPH (IR MPH).
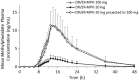
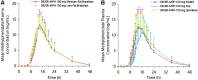
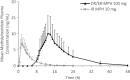
Methods: Three open-label, single-dose, crossover studies were conducted, all with a 7-day washout between treatments. In Study I, 20 subjects received evening-dosed DR/ER-MPH (20 and 100 mg) followed by a medium-fat breakfast; 13 subjects received a subsequent 100-mg dose of DR/ER-MPH followed by a low-fat breakfast. In Study II, 18 subjects were evaluated after receiving evening-dosed DR/ER-MPH (100 mg) under 3 conditions: immediately after a high-fat meal, sprinkled on applesauce, and in a fasted state. In Study III, 11 and 12 subjects received evening-dosed DR/ER-MPH (100 mg) and morning-dosed IR MPH (20 mg), respectively.
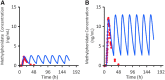
Results: DR/ER-MPH demonstrated dose proportionality between 20- and 100-mg doses. DR/ER-MPH PK parameters were not significantly affected by breakfast content or by sprinkling capsule contents. A high-fat meal immediately preceding evening dosing did not affect total MPH exposure but lowered peak MPH exposure by 14% and 11% versus fasted and sprinkled states, and time to peak exposure was delayed by ∼2.5 hours; these PK differences are unlikely to be clinically significant. Based on multiple-dose simulations using data from Study I, negligible accumulation of DR/ER-MPH was predicted. The relative bioavailability for DR/ER-MPH compared to IR MPH was 73.9%. No serious adverse events (AEs) were reported, and the observed AEs were consistent with MPH. There were no discontinuations in Studies I and III, but three participants withdrew in Study II due to AEs.
Conclusions: Evening-dosed DR/ER-MPH demonstrated dose proportionality and can be administered with or without food. Significant accumulation is unlikely with multiple dosing.
Keywords: attention-deficit/hyperactivity disorder; dose proportionality; food effect; methylphenidate; pharmacokinetics; relative bioavailability.
Figures
References
-
- ALZA Corporation: Concerta Clinical Pharmacology and Biopharmaceutics Review. 2000. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21-121_Concerta_... Accessed June2018
-
- Auiler JF, Liu K, Lynch JM, Gelotte CK: Effect of food on early drug exposure from extended-release stimulants: Results from the CONCERTA, Adderall XR Food Evaluation (CAFE) Study. Curr Med Res Opin 18:311–316, 2002 - PubMed
-
- Childress AC: Methylphenidate HCl for the treatment of ADHD in children and adolescents. Expert Opin Pharmacother 17:1171–1178, 2016 - PubMed
-
- Childress A, Mehrotra S, Gobburu J, McLean A, DeSousa NJ, Incledon B: Single-dose pharmacokinetics of HLD200, a delayed-release and extended-release methylphenidate formulation, in healthy adults and in adolescents and children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 28: 10–18, 2018 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
