Response of collagen matrices under pressure and hydraulic resistance in hydrogels
- PMID: 30810567
- PMCID: PMC6512315
- DOI: 10.1039/c8sm02143k
Response of collagen matrices under pressure and hydraulic resistance in hydrogels
Abstract
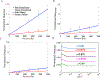
Extracellular matrices in animal tissue are hydrogels mostly made of collagen. In these matrices, collagen fibers are hierarchically assembled and cross-linked to form a porous and elastic material, through which migrating cells can move by either pushing through open matrix pores, or by actively digesting collagen fibers. The influence of matrix mechanical properties on cell behavior is well studied. Less attention has been focused on hydraulic properties of extracellular matrices, and how hydrodynamic flows in these porous hydrogels are influenced by matrix composition and architecture. Here we study the response of collagen hydrogels using rapid changes in the hydraulic pressure within a microfluidic device, and analyze the data using a poroelastic theory. Major poroelastic parameters can be obtained in a single experiment. Results show that depending on the density, porosity, and the degree of geometric confinement, moving micron-sized objects such as cells can experience substantially increased hydraulic resistance (by as much as 106 times) when compared to 2D environments. Therefore, in addition to properties such as mechanical stiffness, the fluidic environment of the cell is also likely to impact cell behavior.
Figures

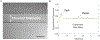
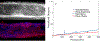

References
Grants and funding
LinkOut - more resources
Full Text Sources

