Nucleophilic addition of phosphorus(iii) derivatives to squaraines: colorimetric detection of transition metal-mediated or thermal reversion
- PMID: 30810572
- PMCID: PMC6857784
- DOI: 10.1039/c9cc01243e
Nucleophilic addition of phosphorus(iii) derivatives to squaraines: colorimetric detection of transition metal-mediated or thermal reversion
Abstract
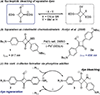
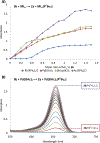
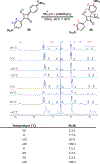
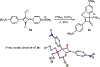
Nucleophilic addition of phosphorus(iii) agents to the electrophilic core of intensely colored squaraine dyes gives a bleached zwitterionic adduct in good to excellent yields (up to 99%) at room temperature. The process can be reversed by adding specific transition metal complexes with high phosphorous(iii) affinity.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures
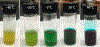
References
-
- Treibs A; Jacob K Angew. Chem. Int. Ed 1965, 4, 694–694.
- Ajayaghosh A Acc. Chem. Res 2005, 38, 449–459. - PubMed
- Avirah RR; Jyothish K; Ramaiah D Org. Lett 2007, 9, 121–124. - PubMed
- Jyothish K; Arun KT; Ramaiah D Org. Lett 2004, 6, 3965–3968. - PubMed
- Ros-Lis JV; Martínez-Máñez R; Sancenón F; Soto J; Spieles M; Rurack K Chem. Eur. J 2008, 14, 10101–10114. - PubMed
-
- Sreejith S; Joseph J; Lin M; Menon NV; Borah P; Ng HJ; Loong YX; Kang Y; Yu SW-K; Zhao Y ACS Nano 2015, 9, 5695–5704. - PubMed
- Harmatys KM; Cole EL; Smith BD Mol. Pharmaceutics 2013, 10, 4263–4271. - PMC - PubMed
- Cole EL; Arunkumar E; Xiao S; Smith BA; Smith BD Org. Biomol. Chem 2012, 10, 5769–5773. - PMC - PubMed
- White AG; Fu N; Leevy WM; Lee J-J; Blasco MA; Smith BD Bioconjugate Chem. 2010, 21, 1297–1304. - PMC - PubMed
- Avirah RR; Jayaram DT; Adarsh N; Ramaiah D Org. Biomol. Chem 2012, 10, 911–920. - PubMed
-
- Prabhakar C; Bhanuprakash K; Rao VJ; Balamuralikrishna M; Rao DN J. Phys. Chem. C 2010, 114, 6077–6089.
- Wang S; Hall L; Diev VV; Haiges R; Wei G; Xiao X; Djurovich PI; Forrest SR; Thompson ME Chem. Mater 2011, 23, 4789–4798.
- Arunkumar E; Ajayaghosh A; Daub JJ Am. Chem. Soc 2005, 127, 3156–3164. - PubMed
-
- Ros-Lis JV; Martinez-Manez R; Soto J Chem. Commun 2002, 2248–2249. - PubMed
-
- Ros-Lis JV; García B; Jiménez D; Martínez-Máñez R; Sancenón F; Soto J; Gonzalvo F; Valldecabres MC J. Am. Chem. Soc 2004, 126, 4064–4065. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

