Conformal Radiation Therapy for Pediatric Ependymoma, Chemotherapy for Incompletely Resected Ependymoma, and Observation for Completely Resected, Supratentorial Ependymoma
- PMID: 30811284
- PMCID: PMC7186586
- DOI: 10.1200/JCO.18.01765
Conformal Radiation Therapy for Pediatric Ependymoma, Chemotherapy for Incompletely Resected Ependymoma, and Observation for Completely Resected, Supratentorial Ependymoma
Abstract
Purpose: The Children's Oncology Group trial ACNS0121 estimated event-free survival (EFS) and overall survival for children with intracranial ependymoma treated with surgery, radiation therapy, and-selectively-with chemotherapy. Treatment was administered according to tumor location, histologic grade, and extent of resection. The impacts of histologic grade, focal copy number gain on chromosome 1q, and DNA methylation profiles were studied for those undergoing surgery and immediate postoperative conformal radiation therapy (CRT).
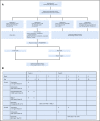
Methods: ACNS0121 included 356 newly diagnosed patients (ages 1 to 21 years). Patients with classic supratentorial ependymoma were observed after gross total resection (GTR). Those undergoing subtotal resection received chemotherapy, second surgery, and CRT. The remaining patients received immediate postoperative CRT after near-total resection or GTR. CRT was administered with a 1.0-cm clinical target volume margin. The cumulative total dose was 59.4 Gy, except for patients who underwent GTR and were younger than age 18 months (who received 54 Gy). Patients were enrolled between October 2003 and September 2007 and were observed for 5 years. Supratentorial tumors were evaluated for RELA fusion; infratentorial tumors, for chromosome 1q gain. Classification of posterior fossa groups A and B was made by methylation profiles.
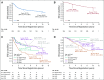
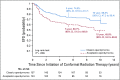
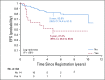
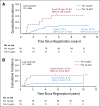
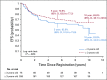
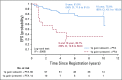
Results: The 5-year EFS rates were 61.4% (95% CI, 34.5% to 89.6%), 37.2% (95% CI, 24.8% to 49.6%), and 68.5% (95% CI, 62.8% to 74.2%) for observation, subtotal resection, and near-total resection/GTR groups given immediate postoperative CRT, respectively. The 5-year EFS rates differed significantly by tumor grade (P = .0044) but not by age, location, RELA fusion status, or posterior fossa A/posterior fossa B grouping. EFS was higher for patients with infratentorial tumors without 1q gain than with 1q gain (82.8% [95% CI, 74.4% to 91.2%] v 47.4% [95% CI, 26.0% to 68.8%]; P = .0013).
Conclusion: The EFS for patients with ependymoma younger than 3 years of age who received immediate postoperative CRT and for older patients is similar. Irradiation should remain the mainstay of care for most subtypes.
Figures

Comment in
-
Pediatric Radiation Therapy-When Too Much Is Not Enough.Int J Radiat Oncol Biol Phys. 2019 Aug 1;104(5):963-966. doi: 10.1016/j.ijrobp.2019.04.022. Int J Radiat Oncol Biol Phys. 2019. PMID: 31327424 No abstract available.
References
-
- Merchant TE, Gilbertson RJ. 2010. Ependymoma, in Estlin EJ, Gilbertson RJ, Wynn RF (eds): Pediatric Hematology and Oncology. Wiley-Blackwell, Oxford, United Kingdom,
-
- Evans AE, Anderson JR, Lefkowitz-Boudreaux IB, et al. Adjuvant chemotherapy of childhood posterior fossa ependymoma: Cranio-spinal irradiation with or without adjuvant CCNU, vincristine, and prednisone—A Childrens Cancer Group study. Med Pediatr Oncol. 1996;27:8–14. - PubMed
-
- Garvin JH, Jr, Selch MT, Holmes E, et al. Phase II study of pre-irradiation chemotherapy for childhood intracranial ependymoma: Children’s Cancer Group protocol 9942—A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2012;59:1183–1189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

