Waterpipe smoke and e-cigarette vapor differentially affect circadian molecular clock gene expression in mouse lungs
- PMID: 30811401
- PMCID: PMC6392409
- DOI: 10.1371/journal.pone.0211645
Waterpipe smoke and e-cigarette vapor differentially affect circadian molecular clock gene expression in mouse lungs
Abstract
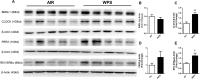
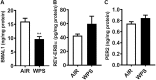
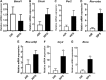
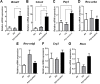
The use of emerging tobacco products, such as waterpipe or hookah and electronic cigarettes (e-cigs), has gained significant popularity and are promoted as safer alternatives to conventional cigarettes. Circadian systems are internal biological oscillations that are considered important regulators of immune functions in mammals. Tobacco induced inflammatory lung diseases frequently exhibit time-of-day/night variation in lung function and symptom severity. We investigated the impact of inhaled e-cig vapor and waterpipe smoke (WPS) on pulmonary circadian molecular clock disruption by determining the changes in expression levels and abundance of core clock component genes (BMAL1, CLOCK) and clock-controlled output genes (Rev-erbα, Per2, Rev-erbβ, Cry2, Rorα) in mouse lungs. We showed that the expression levels of these pulmonary core clock genes and clock-controlled output genes were altered significantly following exposure to WPS (Bmal1, Clock, and Rev-erbα). We further showed a significant yet differential effect on expression levels of core clock and clock-controlled genes (Bmal1, Per2) in the lungs of mice exposed to e-cig vapor containing nicotine. Thus, acute exposure to WPS and e-cig vapor containing nicotine contributes to altered expression of circadian molecular clock genes in mouse lungs, which may have repercussions on lung cellular and biological functions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

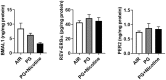

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

