Single-center retrospective study of the effectiveness and toxicity of the oral iron chelating drugs deferiprone and deferasirox
- PMID: 30811439
- PMCID: PMC6392256
- DOI: 10.1371/journal.pone.0211942
Single-center retrospective study of the effectiveness and toxicity of the oral iron chelating drugs deferiprone and deferasirox
Erratum in
-
Correction: Single-center retrospective study of the effectiveness and toxicity of the oral iron chelating drugs deferiprone and deferasirox.PLoS One. 2019 Mar 13;14(3):e0214005. doi: 10.1371/journal.pone.0214005. eCollection 2019. PLoS One. 2019. PMID: 30865729 Free PMC article.
Abstract
Background: Iron overload, resulting from blood transfusions in patients with chronic anemias, has historically been controlled with regular deferoxamine, but its parenteral requirement encouraged studies of orally-active agents, including deferasirox and deferiprone. Deferasirox, licensed by the US Food and Drug Administration in 2005 based upon the results of randomized controlled trials, is now first-line therapy worldwide. In contrast, early investigator-initiated trials of deferiprone were prematurely terminated after investigators raised safety concerns. The FDA declined market approval of deferiprone; years later, it licensed the drug as "last resort" therapy, to be prescribed only if first-line drugs had failed. We undertook to evaluate the long-term effectiveness and toxicities of deferiprone and deferasirox in one transfusion clinic.
Methods and findings: Under an IRB-approved study, we retrospectively inspected the electronic medical records of consented iron-loaded patients managed between 2009 and 2015 at The University Health Network (UHN), Toronto. We compared changes in liver and heart iron, adverse effects and other outcomes, in patients treated with deferiprone or deferasirox.
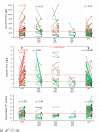
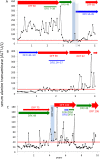
Results: Although deferiprone was unlicensed in Canada, one-third (n = 41) of locally-transfused patients had been switched from first-line, licensed therapies (deferoxamine or deferasirox) to regimens of unlicensed deferiprone. The primary endpoint of monitoring in iron overload, hepatic iron concentration (HIC), increased (worsened) during deferiprone monotherapy (mean 10±2-18±2 mg/g; p < 0.0003), exceeding the threshold for life-threatening complications (15 mg iron/g liver) in 50% patients. During deferasirox monotherapy, mean HIC decreased (improved) (11±1-6±1 mg/g; p < 0.0001). Follow-up HICs were significantly different following deferiprone and deferasirox monotherapies (p < 0.0000002). Addition of low-dose deferoxamine (<40 mg/kg/day) to deferiprone did not result in reductions of HIC to <15 mg/g (baseline 20±4 mg/g; follow-up, 18±4 mg/g; p < 0.2) or in reduction in the proportion of patients with HIC exceeding 15 mg/g (p < 0.2). During deferiprone exposure, new diabetes mellitus, a recognized consequence of inadequate iron control, was diagnosed in 17% patients, most of whom had sustained HICs exceeding 15 mg/g for years; one woman died after 13 months of a regimen of deferiprone and low-dose deferasirox. During deferiprone exposure, serum ALT increased over baseline in 65% patients. Mean serum ALT increased 6.6-fold (p < 0.001) often persisting for years. During deferasirox exposure, mean ALT was unchanged (p < 0.84). No significant differences between treatment groups were observed in the proportions of patients estimated to have elevated cardiac iron.
Conclusions: Deferiprone showed ineffectiveness and significant toxicity in most patients. Combination with low doses of first-line therapies did not improve the effectiveness of deferiprone. Exposure to deferiprone, over six years while the drug was unlicensed, in the face of ineffectiveness and serious toxicities, demands review of the standards of local medical practice. The limited scope of regulatory approval of deferiprone, worldwide, should restrict its exposure to the few patients genuinely unable to tolerate the two effective, first-line therapies.
Conflict of interest statement
Dr. Nancy F. Olivieri led two investigator-initiated trials of deferiprone begun in 1989 (partially funded by Apotex Inc. from 1993) at Toronto’s Hospital for Sick Children and the University of Toronto. In 1995, she identified concerns regarding long-term effectiveness and safety of deferiprone. In 1996, both Toronto trials were terminated abruptly and prematurely, and Dr. Olivieri was threatened with legal remedies should she make concerns public. Dr. Brenda L. Gallie, an independent researcher at The Hospital for Sick Children and the University, was involved directly in all related events over the subsequent decade of legal proceedings. An independent description of the first five years of this controversy provides additional details (Thompson J, Baird P, Downie J. The Olivieri Report: The complete text of the report of the independent committee of inquiry commissioned by the Canadian Association of University Teachers. Toronto: James Lorimer & Co. Publishers; 2001). In 2014, a final settlement respecting all legal matters was mediated between Dr. Olivieri and Apotex. This does not alter the authors' adherence to all PLOS ONE policies on sharing data and materials.
Figures
References
-
- Pippard MJ. Iron loading and chelation therapy In: Weatherall DJ, editor. The thalassemias. Methods in hematology. 6 Edinburgh: Churchill Livingstone; 1983. p. 103–13.
-
- Zurlo MG, De Stefano P, Borgna-Pignatti C, Di Palma A, Piga A, Melevendi C, et al. Survival and causes of death in thalassaemia major. Lancet. 1989;2(8653):27–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

