Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway
- PMID: 30811474
- PMCID: PMC6392299
- DOI: 10.1371/journal.pone.0212513
Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway
Abstract
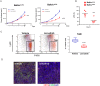
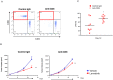
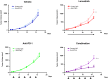
Lenvatinib is a multiple receptor tyrosine kinase inhibitor targeting mainly vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) receptors. We investigated the immunomodulatory activities of lenvatinib in the tumor microenvironment and its mechanisms of enhanced antitumor activity when combined with a programmed cell death-1 (PD-1) blockade. Antitumor activity was examined in immunodeficient and immunocompetent mouse tumor models. Single-cell analysis, flow cytometric analysis, and immunohistochemistry were used to analyze immune cell populations and their activation. Gene co-expression network analysis and pathway analysis using RNA sequencing data were used to identify lenvatinib-driven combined activity with anti-PD-1 antibody (anti-PD-1). Lenvatinib showed potent antitumor activity in the immunocompetent tumor microenvironment compared with the immunodeficient tumor microenvironment. Antitumor activity of lenvatinib plus anti-PD-1 was greater than that of either single treatment. Flow cytometric analysis revealed that lenvatinib reduced tumor-associated macrophages (TAMs) and increased the percentage of activated CD8+ T cells secreting interferon (IFN)-γ+ and granzyme B (GzmB). Combination treatment further increased the percentage of T cells, especially CD8+ T cells, among CD45+ cells and increased IFN-γ+ and GzmB+ CD8+ T cells. Transcriptome analyses of tumors resected from treated mice showed that genes specifically regulated by the combination were significantly enriched for type-I IFN signaling. Pretreatment with lenvatinib followed by anti-PD-1 treatment induced significant antitumor activity compared with anti-PD-1 treatment alone. Our findings show that lenvatinib modulates cancer immunity in the tumor microenvironment by reducing TAMs and, when combined with PD-1 blockade, shows enhanced antitumor activity via the IFN signaling pathway. These findings provide a scientific rationale for combination therapy of lenvatinib with PD-1 blockade to improve cancer immunotherapy.
Conflict of interest statement
Lenvatinib is the marketed product. YK, KT, and YH obtained the patent entitled “Combination of a PD-1 antagonist and a VEGFR/FGFR/RET tyrosine kinase inhibitor for treating cancer” (WO2016140717A1). These do not interfere with our adherence to PLOS ONE policies regarding sharing data and materials.
Figures



References
-
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2016;375(19):1823–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

