Keratin 8 is a potential self-antigen in the coronary artery disease immunopeptidome: A translational approach
- PMID: 30811493
- PMCID: PMC6392305
- DOI: 10.1371/journal.pone.0213025
Keratin 8 is a potential self-antigen in the coronary artery disease immunopeptidome: A translational approach
Abstract
Background: Inflammation is an important risk factor in atherosclerosis, the underlying cause of coronary artery disease (CAD). Unresolved inflammation may result in maladaptive immune responses and lead to immune reactivity to self-antigens. We hypothesized that inflammation in CAD patients would manifest in immune reactivity to self-antigens detectable in soluble HLA-I/peptide complexes in the plasma.
Methods: Soluble HLA-I/peptide complexes were immuno-precipitated from plasma of male acute coronary syndrome (ACS) patients or age-matched controls and eluted peptides were subjected to mass spectrometry to generate the immunopeptidome. Self-peptides were ranked according to frequency and signal intensity, then mouse homologs of selected peptides were used to test immunologic recall in spleens of male apoE-/- mice fed either normal chow or high fat diet. The peptide detected with highest frequency in patient plasma samples and provoked T cell responses in mouse studies was selected for use as a self-antigen to stimulate CAD patient peripheral blood mononuclear cells (PBMCs).
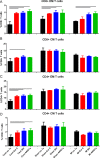
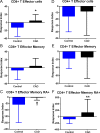
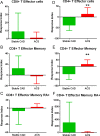
Results: The immunopeptidome profile identified self-peptides unique to the CAD patients. The mouse homologs tested showed immune responses in apoE-/- mice. Keratin 8 was selected for further study in patient PBMCs which elicited T Effector cell responses in CAD patients compared to controls, associated with reduced PD-1 mRNA expression.
Conclusion: An immunopeptidomic strategy to search for self-antigens potentially involved in CAD identified Keratin 8. Self-reactive immune response to Keratin 8 may be an important factor in the inflammatory response in CAD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
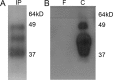

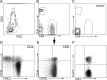
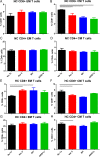


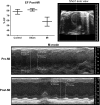


References
-
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017. - PubMed
-
- Lu Y, Zhou S, Dreyer RP, Spatz ES, Geda M, Lorenze NP, et al. Sex Differences in Inflammatory Markers and Health Status Among Young Adults With Acute Myocardial Infarction: Results From the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young Acute Myocardial Infarction Patients) Study. Circ Cardiovasc Qual Outcomes. 2017;10(2): e003470 10.1161/CIRCOUTCOMES.116.003470 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

