Factors Influencing Optical Coherence Tomography Peripapillary Choroidal Thickness: A Multicenter Study
- PMID: 30811523
- PMCID: PMC6392476
- DOI: 10.1167/iovs.18-25407
Factors Influencing Optical Coherence Tomography Peripapillary Choroidal Thickness: A Multicenter Study
Abstract
Purpose: To quantify peripapillary choroidal thickness (PCT) and the factors that influence it in healthy participants who represent the racial and ethnic composition of the U.S. population.
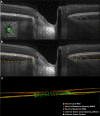
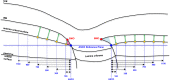
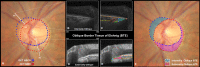
Methods: A total of 362 healthy participants underwent optical coherence tomography (OCT) enhanced depth imaging of the optic nerve head with a 24 radial B-scan pattern aligned to the fovea to Bruch's membrane opening axis. Bruch's membrane, anterior scleral canal opening (ASCO), and the anterior scleral surface were manually segmented. PCT was measured at 100, 300, 500, 700, 900, and 1100 μm from the ASCO globally and within 12 clock-hour sectors. The effects of age, axial length, intraocular pressure, ethnicity, sex, sector, and ASCO area on PCT were assessed by ANOVA and univariable and multivariable regressions.
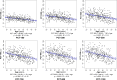
Results: Globally, PCT was thicker further from the ASCO border and thinner with older age, longer axial length, larger ASCO area, European descent, and female sex. Among these effectors, age and axial length explained the greatest proportion of variance. The rate of age-related decline increased further from the ASCO border. Sectorally, the inferior-temporal sectors were thinnest (10.7%-20.0% thinner than the thickest sector) and demonstrated a higher rate of age-related loss (from 15.6% to 20.7% faster) at each ASCO distance.
Conclusions: In healthy eyes, PCT was thinnest in the inferior temporal sectors and thinner PCT was associated with older age, European descent, longer axial length, larger ASCO area, and female sex. Among these associations, age had the strongest influence, and its effect was greatest within the inferior temporal sectors.
Figures



Similar articles
-
Factors Influencing Central Lamina Cribrosa Depth: A Multicenter Study.Invest Ophthalmol Vis Sci. 2018 May 1;59(6):2357-2370. doi: 10.1167/iovs.17-23456. Invest Ophthalmol Vis Sci. 2018. PMID: 29847642 Free PMC article.
-
Assessment of Open-Angle Glaucoma Peripapillary and Macular Choroidal Thickness Using Swept-Source Optical Coherence Tomography (SS-OCT).PLoS One. 2016 Jun 16;11(6):e0157333. doi: 10.1371/journal.pone.0157333. eCollection 2016. PLoS One. 2016. PMID: 27309734 Free PMC article.
-
Peripapillary Scleral Bowing Increases with Age and Is Inversely Associated with Peripapillary Choroidal Thickness in Healthy Eyes.Am J Ophthalmol. 2020 Sep;217:91-103. doi: 10.1016/j.ajo.2020.03.050. Epub 2020 Apr 13. Am J Ophthalmol. 2020. PMID: 32298653 Free PMC article.
-
Peripapillary choroidal thickness variation with age and race in normal eyes.Invest Ophthalmol Vis Sci. 2015 Feb 24;56(3):1872-9. doi: 10.1167/iovs.14-16179. Invest Ophthalmol Vis Sci. 2015. PMID: 25711640 Free PMC article.
-
Macular, choroidal and disc associations across women's reproductive life stages: a scoping review from menarche to post-menopause.Eye (Lond). 2025 Feb;39(3):402-411. doi: 10.1038/s41433-025-03592-w. Epub 2025 Jan 15. Eye (Lond). 2025. PMID: 39814872 Free PMC article.
Cited by
-
Heterogenous thinning of peripapillary tissues occurs early during high myopia development in juvenile tree shrews.Exp Eye Res. 2024 Mar;240:109824. doi: 10.1016/j.exer.2024.109824. Epub 2024 Feb 7. Exp Eye Res. 2024. PMID: 38336167 Free PMC article.
-
Choroidal and Retinal Thicknesses in Healthy Eyes Measured with Ultra-Wide-Field Optical Coherence Tomography.Diagnostics (Basel). 2024 May 28;14(11):1114. doi: 10.3390/diagnostics14111114. Diagnostics (Basel). 2024. PMID: 38893640 Free PMC article.
-
Histologic validation of optical coherence tomography-based three-dimensional morphometric measurements of the human optic nerve head: Methodology and preliminary results.Exp Eye Res. 2021 Apr;205:108475. doi: 10.1016/j.exer.2021.108475. Epub 2021 Jan 28. Exp Eye Res. 2021. PMID: 33516762 Free PMC article.
-
Optical Coherence Tomography Structural Abnormality Detection in Glaucoma Using Topographically Correspondent Rim and Retinal Nerve Fiber Layer Criteria.Am J Ophthalmol. 2020 May;213:203-216. doi: 10.1016/j.ajo.2019.12.020. Epub 2019 Dec 30. Am J Ophthalmol. 2020. PMID: 31899204 Free PMC article.
-
OCT-Detected Optic Nerve Head Neural Canal Direction, Obliqueness, and Minimum Cross-Sectional Area in Healthy Eyes.Am J Ophthalmol. 2019 Dec;208:185-205. doi: 10.1016/j.ajo.2019.05.009. Epub 2019 May 13. Am J Ophthalmol. 2019. PMID: 31095953 Free PMC article.
References
-
- Hayreh SS, Jonas JB, Zimmerman MB. Parapapillary chorioretinal atrophy in chronic high-pressure experimental glaucoma in rhesus monkeys. Invest Ophthalmol Vis Sci. 1998;39:2296–2303. - PubMed
-
- Hayreh SS, Pe'er J, Zimmerman MB. Morphologic changes in chronic high-pressure experimental glaucoma in rhesus monkeys. J Glaucoma. 1999;8:56–71. - PubMed
-
- Vianna JR, Malik R, Danthurebandara VM, et al. Beta and gamma peripapillary atrophy in myopic eyes with and without glaucoma. Invest Ophthalmol Vis Sci. 2016;57:3103–3111. - PubMed
-
- Hayashi K, Tomidokoro A, Lee KY, et al. Spectral-domain optical coherence tomography of beta-zone peripapillary atrophy: influence of myopia and glaucoma. Invest Ophthalmol Vis Sci. 2012;53:1499–1505. - PubMed

