Phosphorylation of GMFγ by c-Abl Coordinates Lamellipodial and Focal Adhesion Dynamics to Regulate Airway Smooth Muscle Cell Migration
- PMID: 30811945
- PMCID: PMC6670026
- DOI: 10.1165/rcmb.2018-0352OC
Phosphorylation of GMFγ by c-Abl Coordinates Lamellipodial and Focal Adhesion Dynamics to Regulate Airway Smooth Muscle Cell Migration
Abstract
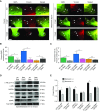
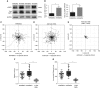
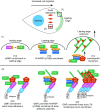
Airway smooth muscle cells require coordinated protrusion and focal adhesion dynamics to migrate properly. However, the signaling cascades that connect these two processes remain incompletely understood. Glia maturation factor (GMF)-γ has been implicated in inducing actin debranching and inhibiting nucleation. In this study, we discovered that GMFγ phosphorylation at Y104 regulates human airway smooth muscle cell migration. Using high-resolution microscopy coupled with three-dimensional object-based quantitative image analysis software, Imaris 9.2.0, phosphomimetic mutant, Y104D-GMFγ, was enriched at nascent adhesions along the leading edge where it recruited activated neural Wiskott-Aldrich syndrome protein (N-WASP; pY256) to promote actin-branch formation, which enhanced lamellipodial dynamics and limited the growth of focal adhesions. Unexpectedly, we found that nonphosphorylated mutant, Y104F-GMFγ, was enriched in growing adhesions where it promoted a linear branch organization and focal adhesion clustering, and recruited zyxin to increase maturation, thus inhibiting lamellipodial dynamics and cell migration. The localization of GMFγ between the leading edge and focal adhesions was dependent upon myosin activity. Furthermore, c-Abl tyrosine kinase regulated the GMFγ phosphorylation-dependent processes. Together, these results unveil the importance of GMFγ phosphorylation in coordinating lamellipodial and focal adhesion dynamics to regulate cell migration.
Keywords: actin cytoskeleton; airway smooth muscle; cell migration; protein phosphorylation.
Figures
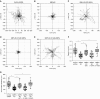
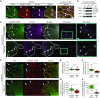
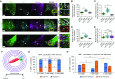
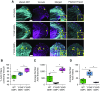
Comment in
-
Orchestrating Airway Smooth Muscle Cell Migration: GMFγ Phosphorylation Is the Key.Am J Respir Cell Mol Biol. 2019 Aug;61(2):136-138. doi: 10.1165/rcmb.2019-0074ED. Am J Respir Cell Mol Biol. 2019. PMID: 30950633 Free PMC article. No abstract available.
References
-
- Gizycki MJ, Adelroth E, Rogers AV, O’Byrne PM, Jeffery PK. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. Am J Respir Cell Mol Biol. 1997;16:664–673. - PubMed
-
- Kaminska M, Foley S, Maghni K, Storness-Bliss C, Coxson H, Ghezzo H, et al. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. J Allergy Clin Immunol. 2009;124:45–51.e1–4. - PubMed
-
- Krause M, Gautreau A. Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol. 2014;15:577–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

