Controlling Epithelial Polarity: A Human Enteroid Model for Host-Pathogen Interactions
- PMID: 30811997
- PMCID: PMC6391775
- DOI: 10.1016/j.celrep.2019.01.108
Controlling Epithelial Polarity: A Human Enteroid Model for Host-Pathogen Interactions
Abstract
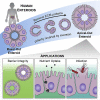
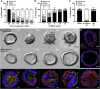
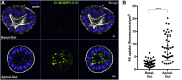
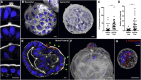
Human enteroids-epithelial spheroids derived from primary gastrointestinal tissue-are a promising model to study pathogen-epithelial interactions. However, accessing the apical enteroid surface is challenging because it is enclosed within the spheroid. We developed a technique to reverse enteroid polarity such that the apical surface everts to face the media. Apical-out enteroids maintain proper polarity and barrier function, differentiate into the major intestinal epithelial cell (IEC) types, and exhibit polarized absorption of nutrients. We used this model to study host-pathogen interactions and identified distinct polarity-specific patterns of infection by invasive enteropathogens. Salmonella enterica serovar Typhimurium targets IEC apical surfaces for invasion via cytoskeletal rearrangements, and Listeria monocytogenes, which binds to basolateral receptors, invade apical surfaces at sites of cell extrusion. Despite different modes of entry, both pathogens exit the epithelium within apically extruding enteroid cells. This model will enable further examination of IECs in health and disease.
Keywords: Listeria; Salmonella; apicobasal polarity; bacterial infection; epithelial organoids; gastrointestinal model; host-pathogen interactions; human enteroids; intestinal epithelial cells.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Basak O., Beumer J., Wiebrands K., Seno H., van Oudenaarden A., Clevers H. Induced quiescence of Lgr5+ stem cells in intestinal organoids enables differentiation of hormone-producing enteroendocrine cells. Cell Stem Cell. 2017;20:177–190.e4. - PubMed
-
- Coburn B., Grassl G.A., Finlay B.B. Salmonella, the host and disease: a brief review. Immunol. Cell Biol. 2007;85:112–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

