Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis
- PMID: 30813108
- PMCID: PMC6361337
- DOI: 10.1136/bmjopen-2018-022577
Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis
Abstract
Objective: To estimate the association between the use of sodium glucose co-transporter-2 (SGLT2) inhibitors and postmarket harms as identified by drug regulatory agencies.
Design: We conducted a systematic review and meta-analysis of randomised controlled trials (RCT). Six large databases were searched from inception to May 2018. Random effects models were used to estimate pooled relative risks (RRs).
Intervention: SGLT2 inhibitors, compared with placebo or active comparators.
Primary outcomes: Acute kidney injury (AKI), diabetic ketoacidosis (DKA), urinary tract infections (UTI), bone fractures and lower limb amputations.
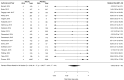
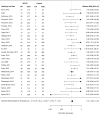
Results: We screened 2418 citations of which 109 were included. Most studies included one of four SGLT2 inhibitors, dapagliflozin, canagliflozin, empagliflozin and ipragliflozin. When compared with placebo, SGLT2 inhibitors were found to be significantly protective against AKI (RR=0.59; 95% CI 0.39 to 0.89; I2=0.0%), while no difference was found for DKA (RR 0.66; 95% CI 0.30 to 1.45, I2=0.0%), UTI (RR 1.02; 95% CI 0.95 to 1.09, I2=0.0%) or bone fracture (RR 0.87; 95% CI 0.69 to 1.09, I2=1.3%). Three studies reported on amputation, with one finding a significant increase risk. No increased risk for either outcome was found when compared with active controls. Subgroup analysis did show an increased risk of UTI with dapagliflozin only (RR 1.21; 95% CI 1.02 to 1.43, I2=0.0%), but no other analysis supported an increased risk of AKI, DKA, UTI or fracture.
Conclusions: Current evidence from RCTs does not suggest an increased risk of harm with SGLT2 inhibitors as a class over placebo or active comparators with respect to AKI, DKA, UTI or fracture. However, wide CIs for many comparisons suggest limited precision, and therefore clinically important adverse events cannot be ruled out. Dapagliflozin, appears to independently increase the risk of UTI, although the mechanism for this intraclass variation in risk is unclear.
Prospero registration number: CRD42016038715.
Keywords: adverse events; epidemiology; therapeutics.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
-
- Canadian Diabetes Association. 2016 Interim update to the guidelines: update to the pharmacologic management of Type 2 diabetes. 2016.
-
- American Diabetes Association. Diabetes Guidelines Summary Recommendations from NDEI 1 2016 American Diabetes Association (ADA) Diabetes Guidelines Summary Recommendations from NDEI. 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
