Deep Learning-Based Framework for In Vivo Identification of Glioblastoma Tumor using Hyperspectral Images of Human Brain
- PMID: 30813245
- PMCID: PMC6412736
- DOI: 10.3390/s19040920
Deep Learning-Based Framework for In Vivo Identification of Glioblastoma Tumor using Hyperspectral Images of Human Brain
Abstract
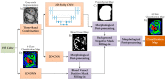
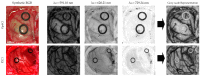
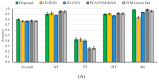
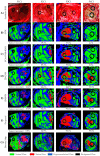
The main goal of brain cancer surgery is to perform an accurate resection of the tumor, preserving as much normal brain tissue as possible for the patient. The development of a non-contact and label-free method to provide reliable support for tumor resection in real-time during neurosurgical procedures is a current clinical need. Hyperspectral imaging is a non-contact, non-ionizing, and label-free imaging modality that can assist surgeons during this challenging task without using any contrast agent. In this work, we present a deep learning-based framework for processing hyperspectral images of in vivo human brain tissue. The proposed framework was evaluated by our human image database, which includes 26 in vivo hyperspectral cubes from 16 different patients, among which 258,810 pixels were labeled. The proposed framework is able to generate a thematic map where the parenchymal area of the brain is delineated and the location of the tumor is identified, providing guidance to the operating surgeon for a successful and precise tumor resection. The deep learning pipeline achieves an overall accuracy of 80% for multiclass classification, improving the results obtained with traditional support vector machine (SVM)-based approaches. In addition, an aid visualization system is presented, where the final thematic map can be adjusted by the operating surgeon to find the optimal classification threshold for the current situation during the surgical procedure.
Keywords: bioinformatics; brain tumor; cancer surgery; deep learning; hyperspectral imaging; image-guided surgery; intraoperative imaging; precision medicine.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures





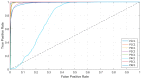
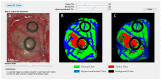
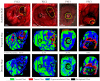

References
-
- Sanai N., Berger M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–764. doi: 10.1227/01.neu.0000318159.21731.cf. - DOI - PubMed
MeSH terms
Grants and funding
- R21CA176684/Foundation for the National Institutes of Health
- R01CA156775/Foundation for the National Institutes of Health
- R01CA204254/Foundation for the National Institutes of Health
- R01HL140325/Foundation for the National Institutes of Health
- ProID2017010164/Agencia Canaria de Investigación, Innovación y Sociedad de la Información
- 618080/Seventh Framework Programme
- TEC2017-86722-C4-1-R/Ministerio de Economía, Industria y Competitividad, Gobierno de España
- 2016 PhD Training Program for Research Staff/Universidad de Las Palmas de Gran Canaria
- POC 2014-2020, Eje 3 Tema Prioritario 74 (85%)/Agencia Canaria de Investigación, Innovación y Sociedad de la Información
LinkOut - more resources
Full Text Sources
Other Literature Sources

