Congenital Lactase Deficiency: Mutations, Functional and Biochemical Implications, and Future Perspectives
- PMID: 30813293
- PMCID: PMC6412902
- DOI: 10.3390/nu11020461
Congenital Lactase Deficiency: Mutations, Functional and Biochemical Implications, and Future Perspectives
Abstract
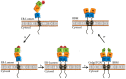
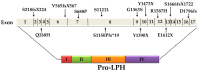
Congenital lactase deficiency (CLD) is a severe autosomal recessive genetic disorder that affects the functional capacity of the intestinal protein lactase-phlorizin hydrolase (LPH). This disorder is diagnosed already during the first few days of the newborn's life due to the inability to digest lactose, the main carbohydrate in mammalian milk. The symptoms are similar to those in other carbohydrate malabsorption disorders, such as congenital sucrase-isomaltase deficiency, and include severe osmotic watery diarrhea. CLD is associated with mutations in the translated region of the LPH gene that elicit loss-of-function of LPH. The mutations occur in a homozygote or compound heterozygote pattern of inheritance and comprise missense mutations as well as mutations that lead to complete or partial truncations of crucial domains in LPH, such as those linked to the folding and transport-competence of LPH and to the catalytic domains. Nevertheless, the identification of the mutations in CLD is not paralleled by detailed genotype/protein phenotype analyses that would help unravel potential pathomechanisms underlying this severe disease. Here, we review the current knowledge of CLD mutations and discuss their potential impact on the structural and biosynthetic features of LPH. We also address the question of whether heterozygote carriers can be symptomatic for CLD and whether genetic testing is needed in view of the severity of the disease.
Keywords: carbohydrate malabsorption; compound heterozygote inheritance; congenital lactase deficiency; lactase-phlorizin hydrolase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Vining E.M., Hadley E.C., Farnham S.A., Schneider E.L. Recommended Dietary Allowances and the Health of the Elderly. N. Engl. J. Med. 1986;314:157–160. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

