The Effect of DNA Topology on Observed Rates of R-Loop Formation and DNA Strand Cleavage by CRISPR Cas12a
- PMID: 30813348
- PMCID: PMC6409811
- DOI: 10.3390/genes10020169
The Effect of DNA Topology on Observed Rates of R-Loop Formation and DNA Strand Cleavage by CRISPR Cas12a
Abstract
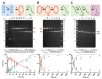
Here we explored the mechanism of R-loop formation and DNA cleavage by type V CRISPR Cas12a (formerly known as Cpf1). We first used a single-molecule magnetic tweezers (MT) assay to show that R-loop formation by Lachnospiraceae bacterium ND2006 Cas12a is significantly enhanced by negative DNA supercoiling, as observed previously with Streptococcus thermophilus DGCC7710 CRISPR3 Cas9. Consistent with the MT data, the apparent rate of cleavage of supercoiled plasmid DNA was observed to be >50-fold faster than the apparent rates for linear DNA or nicked circular DNA because of topology-dependent differences in R-loop formation kinetics. Taking the differences into account, the cleavage data for all substrates can be fitted with the same apparent rate constants for the two strand-cleavage steps, with the first event >15-fold faster than the second. By independently following the ensemble cleavage of the non-target strand (NTS) and target strand (TS), we could show that the faster rate is due to NTS cleavage, the slower rate due to TS cleavage, as expected from previous studies.
Keywords: DNA topology; endonuclease mechanism; rapid reaction kinetics.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
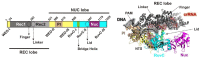


References
-
- Zetsche B., Gootenberg J.S., Abudayyeh O.O., Slaymaker I.M., Makarova K.S., Essletzbichler P., Volz S.E., Joung J., van der Oost J., Regev A., et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

