Wearable Sensors System for an Improved Analysis of Freezing of Gait in Parkinson's Disease Using Electromyography and Inertial Signals
- PMID: 30813411
- PMCID: PMC6412484
- DOI: 10.3390/s19040948
Wearable Sensors System for an Improved Analysis of Freezing of Gait in Parkinson's Disease Using Electromyography and Inertial Signals
Abstract
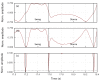
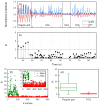
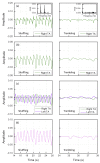
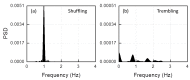
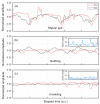
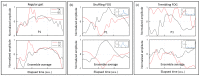
We propose a wearable sensor system for automatic, continuous and ubiquitous analysis of Freezing of Gait (FOG), in patients affected by Parkinson's disease. FOG is an unpredictable gait disorder with different clinical manifestations, as the trembling and the shuffling-like phenotypes, whose underlying pathophysiology is not fully understood yet. Typical trembling-like subtype features are lack of postural adaptation and abrupt trunk inclination, which in general can increase the fall probability. The targets of this work are detecting the FOG episodes, distinguishing the phenotype and analyzing the muscle activity during and outside FOG, toward a deeper insight in the disorder pathophysiology and the assessment of the fall risk associated to the FOG subtype. To this aim, gyroscopes and surface electromyography integrated in wearable devices sense simultaneously movements and action potentials of antagonist leg muscles. Dedicated algorithms allow the timely detection of the FOG episode and, for the first time, the automatic distinction of the FOG phenotypes, which can enable associating a fall risk to the subtype. Thanks to the possibility of detecting muscles contractions and stretching exactly during FOG, a deeper insight into the pathophysiological underpinnings of the different phenotypes can be achieved, which is an innovative approach with respect to the state of art.
Keywords: Parkinson’s disease; gait analysis; inertial signal; sensor fusion; surface electromyography; telemedicine; wearable sensors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

