Gynecological Cancers Translational, Research Implementation, and Harmonization: Gynecologic Cancer InterGroup Consensus and Still Open Questions
- PMID: 30813545
- PMCID: PMC6468728
- DOI: 10.3390/cells8030200
Gynecological Cancers Translational, Research Implementation, and Harmonization: Gynecologic Cancer InterGroup Consensus and Still Open Questions
Abstract
In the era of personalized medicine, the introduction of translational studies in clinical trials has substantially increased their costs, but provides the possibility of improving the productivity of trials with a better selection of recruited patients. With the overall goal of creating a roadmap to improve translational design for future gynecological cancer trials and of defining translational goals, a main discussion was held during a brainstorming day of the Gynecologic Cancer InterGroup (GCIG) Translational Research Committee and overall conclusions are here reported. A particular emphasis was dedicated to the new frontier of the immunoprofiling of gynecological cancers. The discussion pointed out that to maximize patients' benefit, translational studies should be integral to clinical trial design with standardization and optimization of procedures including a harmonization program of Standard Operating Procedures. Pathology-reviewed sample collection should be mandatory and ensured by dedicated funding. Biomarker validation and development should be made public and transparent to ensure rapid progresses with positive outcomes for patients. Guidelines/templates for patients' informed consent are needed. Importantly for the public, recognized goals are to increase the involvement of advocates and to improve the reporting of translational data in a forum accessible to patients.
Keywords: biomarkers definition; gynecological cancers; precision medicine; samples collection; translational studies design.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
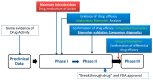
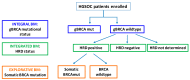
References
-
- Dancey J.E., Dobbin K.K., Groshen S., Jessup J.M., Hruszkewycz A.H., Koehler M., Parchment R., Ratain M.J., Shankar L.K., Stadler W.M., et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin. Cancer Res. 2010;16:1745–1755. doi: 10.1158/1078-0432.CCR-09-2167. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

