Weinreb Amides as Directing Groups for Transition Metal-Catalyzed C-H Functionalizations
- PMID: 30813564
- PMCID: PMC6429370
- DOI: 10.3390/molecules24050830
Weinreb Amides as Directing Groups for Transition Metal-Catalyzed C-H Functionalizations
Abstract
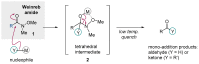
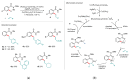
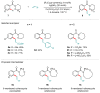
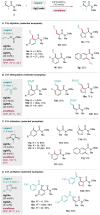
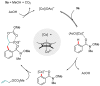
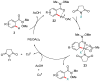
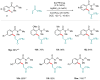
Weinreb amides are a privileged, multi-functional group with well-established utility in classical synthesis. Recently, several studies have demonstrated the use of Weinreb amides as interesting substrates in transition metal-catalyzed C-H functionalization reactions. Herein, we review this part of the literature, including the metal catalysts, transformations explored so far and specific insights from mechanistic studies.
Keywords: C-H functionalization; amides; catalysis; directing groups; transition metals.
Conflict of interest statement
The authors declare that no conflicts of interest exist.
Figures










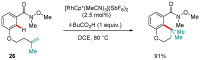





References
-
- Sandtorv A.H. Transition Metal-Catalyzed C-H Activation of Indoles. ACS Catal. 2015;357:2403–2435. doi: 10.1002/adsc.201500374. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

