Colistin Sulfate Chiral Stationary Phase for the Enantioselective Separation of Pharmaceuticals Using Organic Polymer Monolithic Capillary Chromatography
- PMID: 30813595
- PMCID: PMC6429358
- DOI: 10.3390/molecules24050833
Colistin Sulfate Chiral Stationary Phase for the Enantioselective Separation of Pharmaceuticals Using Organic Polymer Monolithic Capillary Chromatography
Abstract
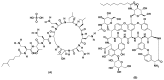
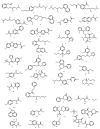
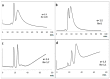
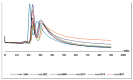
A new functionalized polymer monolithic capillary with a macrocyclic antibiotic, namely colistin sulfate, as chiral selector was prepared via the copolymerization of binary monomer mixtures consisting of glycidyl methacrylate (GMA) and ethylene glycol dimethacrylate (EGDMA) in porogenic solvents namely 1-propanol and 1,4-butanediol, in the presence of azobisiso-butyronitrile (AIBN) as initiator and colistin sulfate. The prepared capillaries were investigated for the enantioselective nano-LC separation of a group of racemic pharmaceuticals, namely, α- and β-blockers, anti-inflammatory drugs, antifungal drugs, norepinephrine-dopamine reuptake inhibitors, catecholamines, sedative hypnotics, antihistaminics, anticancer drugs, and antiarrhythmic drugs. Acceptable separation was achieved for many drugs using reversed phase chromatographic conditions with no separation achieved under normal phase conditions. Colistin sulfate appears to be useful addition to the available macrocyclic antibiotic chiral phases used in liquid chromatography.
Keywords: capillary chromatography; colistin sulfate; enantioselective; encapsulation; monolith; organic polymer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Lin G.Q., Zhang J.G., Cheng J.F. Overview of chirality and chiral drugs. Chiral Drugs Chem. Biol. Action. 2011:14–18.
-
- Ali I., Aboul-Enein H.Y. Chiral Pollutants: Distribution, Toxicity and Analysis by Chromatography and Capillary Electrophoresis. John Wiley & Sons; Chichester, UK: 2005.
-
- Aboul-Enein H., Wainer I. The Impact of Stereochemistry on Drug Development and Use. John Wiley & Sons; New York, NY, USA: 1997. p. 142.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

