Inhibition of NK Reactivity Against Solid Tumors by Platelet-Derived RANKL
- PMID: 30813611
- PMCID: PMC6468810
- DOI: 10.3390/cancers11030277
Inhibition of NK Reactivity Against Solid Tumors by Platelet-Derived RANKL
Abstract
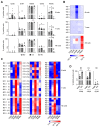
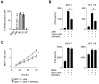
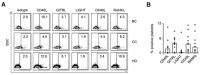
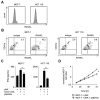
NK cells play an important role in tumor immunosurveillance. Their reactivity is governed by various activating and inhibitory surface receptors, which include several members of the TNF/TNF receptor family. For more than 50 years, it has been recognized that tumor immunosurveillance and in particular NK cell antitumor reactivity is largely influenced by platelets, but the underlying mechanisms remain to be fully elucidated. Here we report that upon activation, which reportedly occurs following interaction with cancer cells, platelets upregulate the TNF family member RANKL. Comparative analysis of the expression of RANK among different NK cell subsets and RANKL on platelets in cancer patients and healthy volunteers revealed a distinct malignant phenotype, and platelet-derived RANKL was found to inhibit the activity of normal NK cells against cancer cells. Notably, NK cell antitumor reactivity could be partially restored by application of denosumab, a RANKL-neutralizing antibody approved for treatment of benign and malignant osteolysis. Together, our data not only unravel a novel mechanism of tumor immune evasion mediated by platelets, but they also provide a functional explanation for the clinical observation that denosumab, beyond protecting from bone loss, may prolong disease-free survival in patients with solid tumors.
Keywords: NK cells; RANK/RANKL; cancer; denosumab; immune evasion; metastasis; platelets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Nieswandt B., Hafner M., Echtenacher B., Mannel D.N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–1300. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

