Lonicera caerulea Extract Attenuates Non-Alcoholic Fatty Liver Disease in Free Fatty Acid-Induced HepG2 Hepatocytes and in High Fat Diet-Fed Mice
- PMID: 30813654
- PMCID: PMC6471428
- DOI: 10.3390/nu11030494
Lonicera caerulea Extract Attenuates Non-Alcoholic Fatty Liver Disease in Free Fatty Acid-Induced HepG2 Hepatocytes and in High Fat Diet-Fed Mice
Abstract
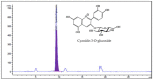
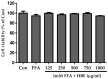
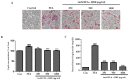
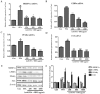
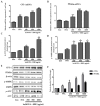
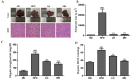
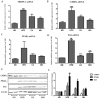
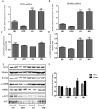
Honeyberry (Lonicera caerulea) has been used for medicinal purposes for thousands of years. Its predominant anthocyanin, cyanidin-3-O-glucoside (C3G), possesses antioxidant and many other potent biological activities. We aimed to investigate the effects of honeyberry extract (HBE) supplementation on HepG2 cellular steatosis induced by free fatty acids (FFA) and in diet-induced obese mice. HepG2 cells were incubated with 1 mM FFA to induce lipid accumulation with or without HBE. Obesity in mice was induced by a 45% high fat diet (HFD) for 6 weeks and subsequent supplementation of 0.5% HBE (LH) and 1% HBE (MH) for 6 weeks. HBE suppressed fatty acid synthesis and ameliorated lipid accumulation in HepG2 cells induced by FFA. Moreover, HBE also decreased lipid accumulation in the liver in the supplemented HBE group (LH, 0.5% or MH, 1%) compared with the control group. The expressions of adipogenic genes involved in hepatic lipid metabolism of sterol regulatory element-binding protein-1 (SREBP-1c), CCAAT/enhancer-binding protein alpha (C/EBPα), peroxisome proliferator-activated receptor gamma (PPARγ), and fatty acid synthase (FAS) were decreased both in the HepG2 cells and in the livers of HBE-supplemented mice. In addition, HBE increased mRNA and protein levels of carnitine palmitoyltransferase (CPT-1) and peroxisome proliferator-activated receptor α (PPARα), which are involved in fatty acid oxidation. Furthermore, HBE treatment increased the phosphorylation of AMP-activated protein kinase (AMPK) and Acetyl CoA Carboxylase (ACC). Honeyberry effectively reduced triglyceride accumulation through down-regulation of hepatic lipid metabolic gene expression and up-regulation of the activation of AMPK and ACC signaling in both the HepG2 cells as well as in livers of diet-induced obese mice. These results suggest that HBE may actively ameliorate non-alcoholic fatty liver disease.
Keywords: HepG2 cells; Honeyberry; free fatty acids; high fat diet; nonalcoholic fatty liver disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Lycopus lucidus Turcz. ex Benth. Attenuates free fatty acid-induced steatosis in HepG2 cells and non-alcoholic fatty liver disease in high-fat diet-induced obese mice.Phytomedicine. 2019 Mar 1;55:14-22. doi: 10.1016/j.phymed.2018.07.008. Epub 2018 Jul 18. Phytomedicine. 2019. PMID: 30668424
-
Psoralea corylifolia L. extract ameliorates nonalcoholic fatty liver disease in free-fatty-acid-incubated HEPG2 cells and in high-fat diet-fed mice.J Food Sci. 2020 Jul;85(7):2216-2226. doi: 10.1111/1750-3841.15166. Epub 2020 Jun 24. J Food Sci. 2020. PMID: 32579753
-
Effects of Eriobotrya japonica Water Extract on Alcoholic and Nonalcoholic Fatty Liver Impairment.J Med Food. 2019 Dec;22(12):1262-1270. doi: 10.1089/jmf.2019.4493. J Med Food. 2019. PMID: 31834842
-
Free radical biology for medicine: learning from nonalcoholic fatty liver disease.Free Radic Biol Med. 2013 Dec;65:952-968. doi: 10.1016/j.freeradbiomed.2013.08.174. Epub 2013 Aug 29. Free Radic Biol Med. 2013. PMID: 23994574 Review.
-
Natural Aldose Reductase Inhibitor: A Potential Therapeutic Agent for Non-alcoholic Fatty Liver Disease.Curr Drug Targets. 2020;21(6):599-609. doi: 10.2174/1389450120666191007111712. Curr Drug Targets. 2020. PMID: 31589122 Review.
Cited by
-
Anthocyanins: Promising Natural Products with Diverse Pharmacological Activities.Molecules. 2021 Jun 22;26(13):3807. doi: 10.3390/molecules26133807. Molecules. 2021. PMID: 34206588 Free PMC article. Review.
-
Study of active components and mechanisms mediating the hypolipidemic effect of Inonotus obliquus polysaccharides.Food Sci Nutr. 2024 Feb 6;12(4):2833-2845. doi: 10.1002/fsn3.3964. eCollection 2024 Apr. Food Sci Nutr. 2024. PMID: 38628208 Free PMC article.
-
Herbal Medicine in the Treatment of Non-Alcoholic Fatty Liver Diseases-Efficacy, Action Mechanism, and Clinical Application.Front Pharmacol. 2020 May 12;11:601. doi: 10.3389/fphar.2020.00601. eCollection 2020. Front Pharmacol. 2020. PMID: 32477116 Free PMC article. Review.
-
The Impact of Anthocyanins and Iridoids on Transcription Factors Crucial for Lipid and Cholesterol Homeostasis.Int J Mol Sci. 2021 Jun 4;22(11):6074. doi: 10.3390/ijms22116074. Int J Mol Sci. 2021. PMID: 34199904 Free PMC article. Review.
-
Mitochondria at the Crossroads: Linking the Mediterranean Diet to Metabolic Health and Non-Pharmacological Approaches to NAFLD.Nutrients. 2025 Mar 30;17(7):1214. doi: 10.3390/nu17071214. Nutrients. 2025. PMID: 40218971 Free PMC article. Review.
References
-
- Hernandez I., Dominguez-Perez M., Bucio L., Souza V., Miranda R.U., Clemens D.L., Gomez-Quiroz L.E., Gutierrez-Ruiz M.C. Free fatty acids enhance the oxidative damage induced by ethanol metabolism in an in vitro model. Food Chem. Toxicol. 2015;76:109–115. doi: 10.1016/j.fct.2014.12.005. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

