Pharmacologic control of CAR-T cell function using dasatinib
- PMID: 30814055
- PMCID: PMC6418502
- DOI: 10.1182/bloodadvances.2018028720
Pharmacologic control of CAR-T cell function using dasatinib
Abstract
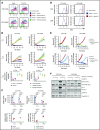
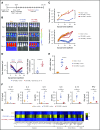
Dasatinib potently and reversibly suppresses CAR-T cell cytotoxicity, cytokine secretion, and proliferation.
Dasatinib could be repurposed as a safety switch to mitigate CAR-mediated toxicity in patients.
Conflict of interest statement
Conflict-of-interest disclosure: C.L.M., E.W.W., and R.C.L. are coinventors on a patent to use dasatinib and other small molecules to modulate CAR function and control CAR-associated toxicity. C.L.M. is a cofounder of Lyell Immunopharma, which is developing CAR-based therapies, and serves as an advisor and consultant for Allogene, Unum Therapeutics, Vor Therapeutics, and GlaxoSmithKline, which are developing CAR-T cell therapies. The remaining authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

