Fibrosing interstitial lung diseases: knowns and unknowns
- PMID: 30814139
- PMCID: PMC9489101
- DOI: 10.1183/16000617.0100-2018
Fibrosing interstitial lung diseases: knowns and unknowns
Abstract
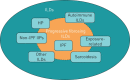
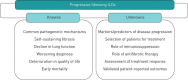
Patients with certain types of fibrosing interstitial lung disease (ILD) are at risk of developing a progressive phenotype characterised by self-sustaining fibrosis, decline in lung function, worsening quality of life, and early mortality. It has been proposed that such progressive fibrosing ILDs, which show commonalities in clinical behaviour and in the pathogenetic mechanisms that drive progressive fibrosis, may be "lumped" together for the purposes of clinical research and, potentially, for treatment. At present, no drugs are approved for the treatment of ILDs other than nintedanib and pirfenidone for the treatment of idiopathic pulmonary fibrosis. For other progressive fibrosing ILDs, the mainstay of drug therapy is immunosuppression. However, it is postulated that, once the response to lung injury in fibrosing ILDs has reached the stage at which fibrosis has become progressive and self-sustaining, targeted antifibrotic therapy would be required to slow disease progression. Nintedanib, an intracellular inhibitor of tyrosine kinases, has shown antifibrotic, anti-inflammatory and vascular remodelling effects in several non-clinical models of fibrosis, irrespective of the trigger for the injury. Ongoing clinical trials will provide insight into the role of antifibrotic treatment with nintedanib or pirfenidone in the management of fibrosing ILDs with a progressive phenotype.
Copyright ©ERS 2019.
Conflict of interest statement
Conflict of interest: V. Cottin reports personal fees from Actelion (for consultancy, lectures and travel to medical meetings), Boehringer Ingelheim (for development of educational presentations, consultancy, lectures and travel to medical meetings), Bayer (for consultancy), Gilead (as a member of an adjudication committee), MSD (for consultancy and travel to medical meetings), Novartis (for consultancy and lectures), Roche (for consultancy, lectures and travel to medical meetings), Sanofi (for consultancy and lectures), Promedior (as chair of the Data and Safety Monitoring Board (DSMB)), Celgene (for the DSMB) and Galapagos (for consultancy and as chair of the DSMB), as well as grants from Boehringer Ingelheim and Roche to his institution, all outside the submitted work. Conflict of interest: L. Wollin is an employee of Boehringer Ingelheim International GmbH. Conflict of interest: A. Fischer reports grants and consultancy fees from Boehringer Ingelheim, and consultancy fees from F. Hoffman La Roche, Bristol-Myers Squibb, GlaxoSmithKline, Pfizer and Genentech, during the conduct of the study. Conflict of interest: M. Quaresma is an employee of Boehringer Ingelheim International GmbH. Conflict of interest: S. Stowasser is an employee of Boehringer Ingelheim International GmbH. Conflict of interest: S. Harari reports grants and personal fees from Roche, Actelion and Boehringer Ingelheim, outside the submitted work. In addition to being investigator in trials involving these companies, relationships include lectures and membership of scientific advisory boards, and grants for research.
Figures
References
-
- Belloli EA, Beckford R, Hadley R, et al. . Idiopathic non-specific interstitial pneumonia. Respirology 2016; 21: 259–268. - PubMed
-
- Hyldgaard C, Bendstrup E, Wells AU, et al. . Unclassifiable interstitial lung diseases: clinical characteristics and survival. Respirology 2017; 22: 494–500. - PubMed
-
- Fischer A, Chartrand S. Assessment and management of connective tissue disease-associated interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis 2015; 32: 2–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
