Effects of blood pressure and lipid lowering on cognition: Results from the HOPE-3 study
- PMID: 30814321
- PMCID: PMC6453765
- DOI: 10.1212/WNL.0000000000007174
Effects of blood pressure and lipid lowering on cognition: Results from the HOPE-3 study
Abstract
Objective: To assess whether long-term treatment with candesartan/hydrochlorothiazide, rosuvastatin, or their combination can slow cognitive decline in older people at intermediate cardiovascular risk.
Methods: The Heart Outcomes Prevention Evaluation-3 (HOPE-3) study was a double-blind, randomized, placebo-controlled clinical trial using a 2 × 2 factorial design. Participants without known cardiovascular disease or need for treatment were randomized to candesartan (16 mg) plus hydrochlorothiazide (12.5 mg) or placebo and to rosuvastatin (10 mg) or placebo. Participants who were ≥70 years of age completed the Digit Symbol Substitution Test (DSST), the modified Montreal Cognitive Assessment, and the Trail Making Test Part B at baseline and study end.
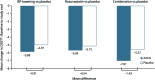
Results: Cognitive assessments were completed by 2,361 participants from 228 centers in 21 countries. Compared with placebo, candesartan/hydrochlorothiazide reduced systolic blood pressure by 6.0 mm Hg, and rosuvastatin reduced low-density lipoprotein cholesterol by 24.8 mg/dL. Participants were followed up for 5.7 years (median), and 1,626 completed both baseline and study-end assessments. Mean participant age was 74 years (SD ±3.5 years); 59% were women; 45% had hypertension; and 24% had ≥12 years of education. The mean difference in change in DSST scores was -0.91 (95% confidence interval [CI] -2.25 to 0.42) for candesartan/hydrochlorothiazide compared with placebo, -0.54 (95% CI -1.88 to 0.80) for rosuvastatin compared with placebo, and -1.43 (95% CI -3.37 to 0.50) for combination therapy vs double placebo. No significant differences were found for other measures.
Conclusions: Long-term blood pressure lowering with candesartan plus hydrochlorothiazide, rosuvastatin, or their combination did not significantly affect cognitive decline in older people.
Clinicaltrialsgov identifier: NCT00468923.
Classification of evidence: This study provides Class II evidence that for older people, candesartan plus hydrochlorothiazide, rosuvastatin, or their combination does not significantly affect cognitive decline.
© 2019 American Academy of Neurology.
Figures

Comment in
-
Does reducing blood pressure and cholesterol provide any HOPE for preventing cognitive decline and dementia?Neurology. 2019 Mar 26;92(13):593-594. doi: 10.1212/WNL.0000000000007175. Epub 2019 Feb 27. Neurology. 2019. PMID: 30814323 No abstract available.
References
-
- World Health Organization. WHO Fact Sheet on Dementia. Geneva: World Health Organization; 2016.
-
- World Health Organization. Dementia Cases Set to Triple by 2050 but Still Largely Ignored.Geneva: World Health Organization; 2016.
-
- Lee Y JHK, Lee KJ, Han G, Kim JL. Association of cognitive status with functional limitation and disability in older adults. Aging Clin Exp Res 2005;17:20–28. - PubMed
-
- Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Social Sci Med 1999;48:445–469. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
