Targeting the NF-κB signaling pathway in chronic tendon disease
- PMID: 30814338
- PMCID: PMC6534967
- DOI: 10.1126/scitranslmed.aav4319
Targeting the NF-κB signaling pathway in chronic tendon disease
Abstract
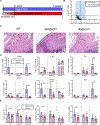
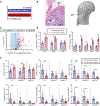
Tendon disorders represent the most common musculoskeletal complaint for which patients seek medical attention; inflammation drives tendon degeneration before tearing and impairs healing after repair. Clinical evidence has implicated the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway as a correlate of pain-free return to function after surgical repair. However, it is currently unknown whether this response is a reaction to or a driver of pathology. Therefore, we aimed to understand the clinically relevant involvement of the NF-κB pathway in tendinopathy, to determine its potential causative roles in tendon degeneration, and to test its potential as a therapeutic candidate. Transcriptional profiling of early rotator cuff tendinopathy identified increases in NF-κB signaling, including increased expression of the regulatory serine kinase subunit IKKβ, which plays an essential role in inflammation. Using cre-mediated overexpression of IKKβ in tendon fibroblasts, we observed degeneration of mouse rotator cuff tendons and the adjacent humeral head. These changes were associated with increases in proinflammatory cytokines and innate immune cells within the joint. Conversely, genetic deletion of IKKβ in tendon fibroblasts partially protected mice from chronic overuse-induced tendinopathy. Furthermore, conditional knockout of IKKβ improved outcomes after surgical repair, whereas overexpression impaired tendon healing. Accordingly, targeting of the IKKβ/NF-κB pathway in tendon stromal cells may offer previously unidentified therapeutic approaches in the management of human tendon disorders.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures



Comment in
-
Targeting NF-κB in tendinopathy.Nat Rev Rheumatol. 2019 May;15(5):251. doi: 10.1038/s41584-019-0206-x. Nat Rev Rheumatol. 2019. PMID: 30914773 No abstract available.
References
-
- Jordan KP, Jöud A, Bergknut C, Croft P, Edwards JJ, Peat G, Petersson IF, Turkiewicz A, Wilkie R, Englund M, International comparisons of the consultation prevalence of musculoskeletal conditions using population-based healthcare data from England and Sweden. Ann. Rheum. Dis. 73, 212–218 (2014). - PMC - PubMed
-
- Riley G, Tendinopathy—From basic science to treatment. Nat. Clin. Pract. Rheumatol. 4, 82–89 (2008). - PubMed
-
- Beard DJ, Rees JL, Cook JA, Rombach I, Cooper C, Merritt N, Shirkey BA, Donovan JL, Gwilym S, Savulescu J, Moser J, Gray A, Jepson M, Tracey I, Judge A, Wartolowska K, Carr AJ, Ahrens P, Baldwick C, Brinsden M, Brownlow H, Burton D, Butt MS, Carr A, Charalambous CP, Conboy V, Dennell L, Donaldson O, Drew S, Dwyer A, Gidden D, Hallam P, Kalogrianitis S, Kelly C, Kulkarni R, Matthews T, McBirnie J, Patel V, Peach C, Roberts C, Robinson D, Rosell P, Rossouw D, Senior C, Singh B, Sjolin S, Taylor G, Venkateswaran B, Woods D, Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): A multicentre, pragmatic, parallel group, placebo-controlled, three-group, randomised surgical trial. Lancet 391, 329–338 (2018). - PMC - PubMed
-
- Ketola S, Lehtinen JT, Arnala I, Arthroscopic decompression not recommended in the treatment of rotator cuff tendinopathy: A final review of a randomised controlled trial at a minimum follow-up often years. Bone Joint J. 99-B, 799–805 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

