Over-expression of FSIP1 promotes breast cancer progression and confers resistance to docetaxel via MRP1 stabilization
- PMID: 30814489
- PMCID: PMC6393503
- DOI: 10.1038/s41419-018-1248-8
Over-expression of FSIP1 promotes breast cancer progression and confers resistance to docetaxel via MRP1 stabilization
Abstract
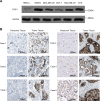
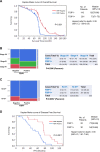
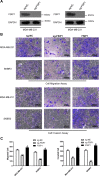
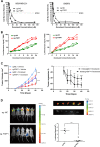
Fibrous sheath-interacting protein 1 (FSIP1) functions centrally in breast carcinogenesis and progression, although its exact role remains to be clarified. Therefore, we sought to establish a correlation between the clinico-pathological features of breast cancer and FSIP1 expression in breast cancer tissues, as well as to validate its role in tumor progression and chemo-resistance. We analyzed FSIP1 expression in the breast cancer and para-tumor tissues by immunohistochemistry. We performed MTT, Caspase-Glo 3/7 Assay, Annexin V staining, wound healing and trans-well assays to evaluate cellular apoptosis, proliferation, migration and invasion in FSIP1 knockout and wild-type breast cancer cell lines. Additionally, we examined the effects of FSIP1 on docetaxel sensitivity in a nude mice model transplanted with control or FSIP1 knockout breast cancer cells, and also evaluate its role in tumor metastasis. FSIP1 and MRP1 interaction was determined by co-immunoprecipitation and mass spectrometry. We found that breast cancer cells and tissues consistently demonstrated elevated FSIP1 expressions, which correlated with poor overall survival. Notably, patients with high FSIP1 expression in their tumors undergoing docetaxel neoadjuvant chemotherapy had shorter disease-free survival. FSIP1 knockout in breast cancer cells significantly increased their sensitivity to docetaxel both in vitro and in vivo. Mechanistically, FSIP1 bound to the multidrug resistance protein 1 (MRP1) and stabilized it, and knocking out FSIP1 decreased MRP1 expression and increased cellular docetaxel accumulation. In sum, FSIP1 promotes breast carcinogenesis and mediates docetaxel resistance, and may serve as a novel target in the development of breast cancer therapies.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
All experiments and protocols adhered to ethical standards outlined in the Declaration of Helsinki and approved by the ethics committee of Harbin Medical University Cancer Hospital in Harbin, China. Consent was obtained from all patients prior to tumor sample collection.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

