Regulation of senescence escape by TSP1 and CD47 following chemotherapy treatment
- PMID: 30814491
- PMCID: PMC6393582
- DOI: 10.1038/s41419-019-1406-7
Regulation of senescence escape by TSP1 and CD47 following chemotherapy treatment
Abstract
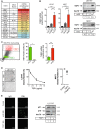
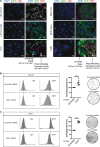
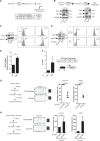
Senescence is a tumor-suppressive mechanism induced by telomere shortening, oncogenes, or chemotherapy treatment. Although it is clear that this suppressive pathway leads to a permanent arrest in primary cells, this might not be the case in cancer cells that have inactivated their suppressive pathways. We have recently shown that subpopulations of cells can escape chemotherapy-mediated senescence and emerge as more transformed cells that induce tumor formation, resist anoikis, and are more invasive. In this study, we characterized this emergence and showed that senescent cells favor tumor growth and metastasis, in vitro and in vivo. Senescence escape was regulated by secreted proteins produced during emergence. Among these, we identified thrombospondin-1 (TSP1), a protein produced by senescent cells that prevented senescence escape. Using SWATH quantitative proteomic analysis, we found that TSP1 can be detected in the serum of patients suffering from triple-negative breast cancer and that its low expression was associated with treatment failure. The results also indicate that senescence escape is explained by the emergence of CD47low cells that express a reduced level of CD47, the TSP1 receptor. The results show that CD47 expression is regulated by p21waf1. The cell cycle inhibitor was sufficient to maintain senescence since its downregulation in senescent cells increased cell emergence. This leads to the upregulation of Myc, which then binds to the CD47 promoter to repress its expression, allowing the generation of CD47low cells that escape the suppressive arrest. Altogether, these results uncovered a new function for TSP1 and CD47 in the control of chemotherapy-mediated senescence.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876–1883. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

