Viral pathogens hitchhike with insect sperm for paternal transmission
- PMID: 30814506
- PMCID: PMC6393494
- DOI: 10.1038/s41467-019-08860-4
Viral pathogens hitchhike with insect sperm for paternal transmission
Abstract
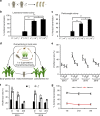
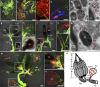
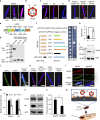
Arthropod-borne viruses (arboviruses) can be maternally transmitted by female insects to their offspring, however, it is unknown whether male sperm can directly interact with the arbovirus and mediate its paternal transmission. Here we report that an important rice arbovirus is paternally transmitted by the male leafhoppers by hitchhiking with the sperm. The virus-sperm binding is mediated by the interaction of viral capsid protein and heparan sulfate proteoglycan on the sperm head surfaces. Mating experiments reveal that paternal virus transmission is more efficient than maternal transmission. Such paternal virus transmission scarcely affects the fitness of adult males or their offspring, and plays a pivotal role in maintenance of viral population during seasons unfavorable for rice hosts in the field. Our findings reveal that a preferred mode of vertical arbovirus transmission has been evolved by hitchhiking with insect sperm without disturbing sperm functioning, facilitating the long-term viral epidemic and persistence in nature.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

