Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases
- PMID: 30814510
- PMCID: PMC6393468
- DOI: 10.1038/s41419-019-1453-0
Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases
Abstract
The pressure towards innovation and creation of new model systems in regenerative medicine and cancer research has fostered the development of novel potential therapeutic applications. Kidney injuries provoke a high request of organ transplants making it the most demanding system in the field of regenerative medicine. Furthermore, renal cancer frequently threaten patients' life and aggressive forms still remain difficult to treat. Ethical issues related to the use of embryonic stem cells, has fueled research on adult, patient-specific pluripotent stem cells as a model for discovery and therapeutic development, but to date, normal and cancerous renal experimental models are lacking. Several research groups are focusing on the development of organoid cultures. Since organoids mimic the original tissue architecture in vitro, they represent an excellent model for tissue engineering studies and cancer therapy testing. We established normal and tumor renal cell carcinoma organoids previously maintained in a heterogeneous multi-clone stem cell-like enriching medium. Starting from adult normal kidney specimens, we were able to isolate and propagate organoid 3D-structures composed of both differentiated and undifferentiated cells while expressing nephron specific markers. Furthermore, we were capable to establish organoids derived from cancer tissues although with a success rate inferior to that of their normal counterpart. Cancer cultures displayed epithelial and mesenchymal phenotype while retaining tumor specific markers. Of note, tumor organoids recapitulated neoplastic masses when orthotopically injected into immunocompromised mice. Our data suggest an innovative approach of long-term establishment of normal- and cancer-derived renal organoids obtained from cultures of fleshly dissociated adult tissues. Our results pave the way to organ replacement pioneering strategies as well as to new models for studying drug-induced nephrotoxicity and renal diseases. Along similar lines, deriving organoids from renal cancer patients opens unprecedented opportunities for generation of preclinical models aimed at improving therapeutic treatments.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
All patients samples were collected and handled in the study following the ethical internationally recognized guide lines and the project was approved by the Ethics Committee of IRE (Prot. CE/44113; 27/11/2012/RS258/12). All animal procedures were performed according to the protocol approved by the Istituto Superiore di Sanità Animal Care Committee (DM N° 228/2009).
Figures

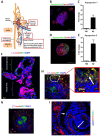
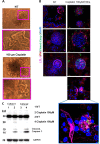


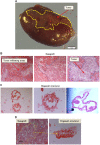
Similar articles
-
Organoids: Avatars for Personalized Medicine.Keio J Med. 2019;68(4):95. doi: 10.2302/kjm.68-006-ABST. Keio J Med. 2019. PMID: 31875622
-
Stem cell-derived organoids and their application for medical research and patient treatment.J Mol Med (Berl). 2017 Jul;95(7):729-738. doi: 10.1007/s00109-017-1531-7. Epub 2017 Apr 8. J Mol Med (Berl). 2017. PMID: 28391362 Review.
-
Importance of organoids for personalized medicine.Per Med. 2018 Nov;15(6):461-465. doi: 10.2217/pme-2018-0071. Epub 2018 Nov 12. Per Med. 2018. PMID: 30418092 Review.
-
Organoids for Drug Discovery and Personalized Medicine.Annu Rev Pharmacol Toxicol. 2019 Jan 6;59:447-462. doi: 10.1146/annurev-pharmtox-010818-021108. Epub 2018 Aug 16. Annu Rev Pharmacol Toxicol. 2019. PMID: 30113875 Review.
-
Obstacles in Renal Regenerative Medicine: Metabolic and Epigenetic Parallels Between Cellular Reprogramming and Kidney Cancer Oncogenesis.Eur Urol Focus. 2019 Mar;5(2):250-261. doi: 10.1016/j.euf.2017.08.003. Epub 2017 Sep 11. Eur Urol Focus. 2019. PMID: 28847686 Review.
Cited by
-
Cancer research using organoid technology.J Mol Med (Berl). 2021 Apr;99(4):501-515. doi: 10.1007/s00109-020-01990-z. Epub 2020 Oct 14. J Mol Med (Berl). 2021. PMID: 33057820 Free PMC article. Review.
-
Organoid Transplantation Can Improve Reproductive Prognosis by Promoting Endometrial Repair in Mice.Int J Biol Sci. 2022 Mar 21;18(6):2627-2638. doi: 10.7150/ijbs.69410. eCollection 2022. Int J Biol Sci. 2022. PMID: 35414792 Free PMC article.
-
Emerging organoid-immune co-culture models for cancer research: from oncoimmunology to personalized immunotherapies.J Immunother Cancer. 2023 May;11(5):e006290. doi: 10.1136/jitc-2022-006290. J Immunother Cancer. 2023. PMID: 37220953 Free PMC article. Review.
-
Establishment of a ccRCC patient-derived chick chorioallantoic membrane model for drug testing.Front Med (Lausanne). 2022 Oct 6;9:1003914. doi: 10.3389/fmed.2022.1003914. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36275794 Free PMC article.
-
Renal tumouroids: challenges of manufacturing 3D cultures from patient derived primary cells.J Cell Commun Signal. 2022 Dec;16(4):637-648. doi: 10.1007/s12079-022-00666-2. Epub 2022 Jan 31. J Cell Commun Signal. 2022. PMID: 35102500 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

