Novel Chitohexaose Analog Protects Young and Aged mice from CLP Induced Polymicrobial Sepsis
- PMID: 30814582
- PMCID: PMC6393422
- DOI: 10.1038/s41598-019-38731-3
Novel Chitohexaose Analog Protects Young and Aged mice from CLP Induced Polymicrobial Sepsis
Abstract
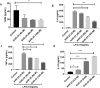
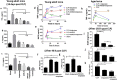
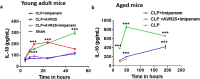
In Gram-negative bacterial sepsis, production of excess pro-inflammatory cytokines results in hyperinflammation and tissue injury. Anti-inflammatory cytokines such as IL-10 inhibit inflammation and enhance tissue healing. Here, we report a novel approach to treat septicemia associated with intra-abdominal infection in a murine model by delicately balancing pro- and anti-inflammatory cytokines. A novel oligosaccharide compound AVR-25 selectively binds to the TLR4 protein (IC50 = 0.15 µM) in human peripheral blood monocytes and stimulates IL-10 production. Following the cecal ligation and puncture (CLP) procedure, intravenous dosing of AVR-25 (10 mg/kg, 6-12 h post-CLP) alone and in combination with antibiotic imipenem protected both young adult (10-12 week old) and aged (16-18 month old) mice against polymicrobial infection, organ dysfunction, and death. Proinflammatory cytokines (TNF-α, MIP-1, i-NOS) were decreased significantly and restoration of tissue damage was observed in all organs. A decrease in serum C-reactive protein (CRP) and bacterial colony forming unit (CFU) confirmed improved bacterial clearance. Together, these findings demonstrate the therapeutic ability of AVR-25 to mitigate the storm of inflammation and minimize tissue injury with high potential for adjunctive therapy in intra-abdominal sepsis.
Conflict of interest statement
The work done by M.E.P. and V.B. was funded by AyuVis Research as subaward from the NIH grant. S.M.O. and S.K.P. received compensation from AyuVis as a member of the scientific advisory board and as consultant, respectively. S.B. received compensation as a contract employee from AyuVis Research. No competing financial or non-financial competing interest is noted for S.A., J.S.S., and B.A.
Figures




References
-
- Holzheimer, R. G., Schein, M. & Wittmann, D. H. Inflammatory response in peritoneal exudate and plasma of patients undergoing planned relaparotomy for severe secondary peritonitis. Archives of surgery (Chicago, Ill.: 1960) 130, 1314–1319, discussion1319–1320 (1995). - PubMed
-
- Cavaillon JM, Munoz C, Fitting C, Misset B, Carlet J. Circulating cytokines: the tip of the iceberg? Circulatory shock. 1992;38:145–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

