Quantitative assessment of regional variation in tissue clearing efficiency using optical coherence tomography (OCT) and magnetic resonance imaging (MRI): A feasibility study
- PMID: 30814611
- PMCID: PMC6393517
- DOI: 10.1038/s41598-019-39634-z
Quantitative assessment of regional variation in tissue clearing efficiency using optical coherence tomography (OCT) and magnetic resonance imaging (MRI): A feasibility study
Abstract
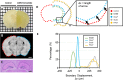
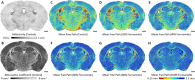
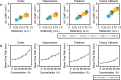
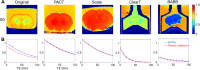
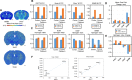
Tissue clearing has gained attention as a pioneering research tool for imaging of large tissue samples. This technique improves light transmission by reducing light scattering within tissues, either by removing lipids or by replacing water with a high refractive index solution. Although various clearing techniques have been developed, quantitative assessments on clearing efficacy depending on tissue properties are rare. In this study, we developed the quantitative mapping of regional clearing efficacy using mean free path in optical coherence tomography (OCT) and proton density in magnetic resonance imaging (MRI), and demonstrated its feasibility in the brain sample with four representative clearing techniques (benzyl alcohol and benzyl benzoate [BABB], ClearT, Scale, and passive CLARITY technique [PACT]). BABB (solvent-based clearing), involving both refractive index matching and lipid removal, exhibited best optical clearing performance with the highest proton density reduction both in gray and white matter. Lipid-removing techniques such as Scale (aqueous hyperhydration) and PACT (hydrogel embedding) showed higher clearing efficiency in white matter than gray matter in accordance with larger proton density increase in white matter. For ClearT (aqueous-based simple immersion), we observed lowest clearing efficiency in the white matter as well as poor lipid removal reflected in low proton density reduction. Our results showed the feasibility of the regional mapping of clearing efficacy and correlating optical transparency and proton density changes using OCT and MRI from existing tissue clearing techniques. This novel quantitative mapping of clearing efficacy depending on tissue types and clearing methods may be helpful in the development of optimized clearing methods for different biological samples.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

