Motor Recovery after Transplantation of Bone Marrow Mesenchymal Stem Cells in Rat Models of Spinal Cord Injury
- PMID: 30814821
- PMCID: PMC6388433
- DOI: 10.1159/000487069
Motor Recovery after Transplantation of Bone Marrow Mesenchymal Stem Cells in Rat Models of Spinal Cord Injury
Abstract
Background: Neuronal tissue has a limited potential to self-renew or get repaired after damage. Cell therapies using stem cells are promising approaches for the treatment of central nervous system (CNS) injuries. However, the clinical use of embryonic stem cells is limited by ethical concerns and other scientific consequences. Bone marrow mesenchymal stromal cells (BM-MSC) could represent an alternative source of stem cells for replacement therapy. Indeed, many studies have demonstrated that MSCs can give rise to neuronal cells as well as many tissue-specific cell phenotypes.
Purpose: Motor recovery by transplantation of bone marrow MSCs in rat models of spinal cord injury (SCI).
Methods: Bone marrow was collected from the femur of albino Wistar rats. MSCs were separated using the Ficoll-Paque density gradient method and cultured in Dulbecco's Modified Eagle Medium supplemented with 20% fetal bovine serum. Cultured MSC was characterized by immunohistochemistry and flow cytometry and neuronal-induced cells were further characterized for neural markers. Cultured MSCs were transplanted into the experimentally injured spinal cord of Wistar rats. Control (injured, but without cell transplantation) and transplanted rats were followed up to 8 weeks, analyzed using the Basso, Beattie, Bresnahan (BBB) scale and electromyography (EMG) for behavioral and physiological status of the injured spinal cord. Finally, the tissue was evaluated histologically.
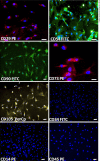
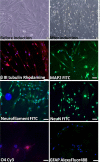
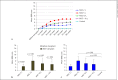
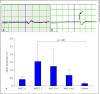
Results: Rat MSCs expressed positivity for a panel of MSC markers CD29, CD54, CD90, CD73, and CD105, and negativity for hematopoietic markers CD34, CD14, and CD45. In vitro neuronal transdifferentiated MSCs express positivity for β III tubulin, MAP2, NF, NeuN, Nav1.1, oligodendrocyte (O4), and negativity for glial fibrillary acid protein. All the treated groups show promising hind-limb motor recovery BBB score, except the control group. There was increased EMG amplitude in treated groups as compared to the control group. Green fluorescent protein (GFP)-labeled MSC survived and differentiated into neurons in the injured spinal cord, which is responsible for functional recovery.
Conclusion: Our results demonstrate that BM-MSC has the potential to repair the injured cord in rat models of SCI. Thus, BM-MSC appears to be a promising candidate for cell-based therapy in CNS injury.
Keywords: Basso, Beattie, Bresnahan score; Electromyography; Mesenchymal stem cells; Spinal cord injury; Transplantation.
Figures





Similar articles
-
Functional Recovery Following the Transplantation of Olfactory Ensheathing Cells in Rat Spinal Cord Injury Model.Asian Spine J. 2018 Dec;12(6):998-1009. doi: 10.31616/asj.2018.12.6.998. Epub 2018 Oct 16. Asian Spine J. 2018. PMID: 30322257 Free PMC article.
-
Globose basal cells for spinal cord regeneration.Neural Regen Res. 2017 Nov;12(11):1895-1904. doi: 10.4103/1673-5374.219052. Neural Regen Res. 2017. PMID: 29239337 Free PMC article.
-
Novel therapeutic approach to slow down the inflammatory cascade in acute/subacute spinal cord injury: Early immune therapy with lipopolysaccharide enhanced neuroprotective effect of combinational therapy of granulocyte colony-stimulating factor and bone-marrow mesenchymal stem cell in spinal cord injury.Front Cell Neurosci. 2022 Nov 23;16:993019. doi: 10.3389/fncel.2022.993019. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36505513 Free PMC article.
-
A combination of mesenchymal stem cells and scaffolds promotes motor functional recovery in spinal cord injury: a systematic review and meta-analysis.J Neurosurg Spine. 2019 Nov 1;32(2):269-284. doi: 10.3171/2019.8.SPINE19201. Print 2020 Feb 1. J Neurosurg Spine. 2019. PMID: 31675724
-
Stem cell-derived exosome treatment for acute spinal cord injury: a systematic review and meta-analysis based on preclinical evidence.Front Neurol. 2025 Jan 24;16:1447414. doi: 10.3389/fneur.2025.1447414. eCollection 2025. Front Neurol. 2025. PMID: 39926016 Free PMC article.
Cited by
-
The Sonic Hedgehog Pathway Modulates Survival, Proliferation, and Differentiation of Neural Progenitor Cells under Inflammatory Stress In Vitro.Cells. 2022 Feb 20;11(4):736. doi: 10.3390/cells11040736. Cells. 2022. PMID: 35203385 Free PMC article.
-
Combination of Chemical and Neurotrophin Stimulation Modulates Neurotransmitter Receptor Expression and Activity in Transdifferentiating Human Adipose Stromal Cells.Stem Cell Rev Rep. 2019 Dec;15(6):851-863. doi: 10.1007/s12015-019-09915-1. Stem Cell Rev Rep. 2019. PMID: 31529274
-
[Research progress of hydrogel combined with mesenchymal stem cells in the treatment of spinal cord injury].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2021 Aug 25;38(4):805-811. doi: 10.7507/1001-5515.202005055. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2021. PMID: 34459182 Free PMC article. Review. Chinese.
-
Enhanced therapeutic potential of Flotillins-modified MenSCs by improve the survival, proliferation and migration.Mol Biol Rep. 2024 May 25;51(1):680. doi: 10.1007/s11033-024-09624-0. Mol Biol Rep. 2024. PMID: 38796595
-
Mesenchymal stem cells in the treatment of spinal cord injury: Mechanisms, current advances and future challenges.Front Immunol. 2023 Feb 24;14:1141601. doi: 10.3389/fimmu.2023.1141601. eCollection 2023. Front Immunol. 2023. PMID: 36911700 Free PMC article. Review.
References
-
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. - PubMed
-
- Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254–264. - PubMed
-
- Kwon BK, Borisoff JF, Tetzlaff W. Molecular targets for therapeutic intervention after spinal cord injury. Mol Interv. 2002;2:244–258. - PubMed
-
- Furuya T, Hashimoto M, Koda M, Okawa A, Murata A, Takahashi K, et al. Treatment of rat spinal cord injury with a Rho-kinase inhibitor and bone marrow stromal cell transplantation. Brain Res. 2009;1295:192–202. - PubMed
-
- Parr AM, Kulbatski I, Wang XH, Keating A, Tator CH. Fate of transplanted adult neural stem/progenitor cells and bone marrow-derived mesenchymal stromal cells in the injured adult rat spinal cord and impact on functional recovery. Surg Neurol. 2008;70:600–607. discussion 607. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

