Treating More with Less: Effectiveness and Event Outcomes of Antituberculosis Fixed-dose Combination Drug versus Separate-drug Formulation (Ethambutol, Isoniazid, Rifampicin and Pyrazinamide) for Pulmonary Tuberculosis Patients in Real-world Clinical Practice
- PMID: 30814828
- PMCID: PMC6380106
- DOI: 10.4103/jgid.jgid_50_18
Treating More with Less: Effectiveness and Event Outcomes of Antituberculosis Fixed-dose Combination Drug versus Separate-drug Formulation (Ethambutol, Isoniazid, Rifampicin and Pyrazinamide) for Pulmonary Tuberculosis Patients in Real-world Clinical Practice
Abstract
Introduction: Conventionally, a combination of four separate drugs (ethambutol, isoniazid, rifampicin, and pyrazinamide [EHRZ]) is the first-line pharmacotherapy for pulmonary tuberculosis (TB). In recent years, fixed-dose combination (FDC) formulation, where a single tablet contains the active ingredients of four aforementioned drugs, is gaining popularity due to its ease of administration.
Objective: To compare the real-world effectiveness of EHRZ and FDC treatment groups on a cohort registry by investigating the sputum conversion rate and treatment outcomes of both groups.
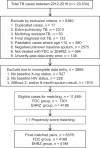
Methods: A total of 11,489 patients' data were extracted from the Sabah TB registry between January 2012 and June 2016, including EHRZ (n = 4188) and FDC (n = 7301) patients. Then, 1:1 propensity score matching was adopted to reduce the baseline bias. Caliper matching was conducted with maximum tolerance score set at 0.001. Confounders included in the propensity score matching were gender, nationality, diabetes, HIV status, smoking status, and chest X-ray status. Successful matching provided 4188 matched pairs (n = 8376) for final analysis.
Results: In this matched cohort of 4188 pairs, the 2-month sputum conversion rate of FDC group was significantly higher than the EHRZ group (96.3% vs. 94.3%; P < 0.001) whereas 6-month sputum conversion of both groups showed no significant difference. Treatment outcomes such as noncompliance rate, failure rate, and success rate have no significant difference (P > 0.05) in both the treatment groups. There was an incidental finding of reduced death rate among FDC group compared to the EHRZ group (0.2% vs. 0.5%; P = 0.034).
Conclusion: The FDC formulation has better sputum conversion rate at 2 months compared to conventional EHRZ regime as separate-drug formulation. It was also observed that FDC has a slight protective effect against all-cause death among TB patients. This protective effect of FDC, however, still needs to be proven further.
Keywords: Akurit-4; fixed-dose combination; intensive phase; propensity score; pulmonary tuberculosis; tuberculosis.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Treatment outcomes of fixed-dose combination versus separate tablet regimens in pulmonary tuberculosis patients with or without diabetes in Qatar.BMC Infect Dis. 2017 Feb 2;17(1):118. doi: 10.1186/s12879-017-2231-1. BMC Infect Dis. 2017. PMID: 28152986 Free PMC article.
-
Fixed-dose combination associated with faster time to smear conversion compared to separate tablets of anti-tuberculosis drugs in patients with poorly controlled diabetes and pulmonary tuberculosis in Qatar.BMC Infect Dis. 2018 Aug 8;18(1):384. doi: 10.1186/s12879-018-3309-0. BMC Infect Dis. 2018. PMID: 30089476 Free PMC article.
-
API TB Consensus Guidelines 2006: Management of pulmonary tuberculosis, extra-pulmonary tuberculosis and tuberculosis in special situations.J Assoc Physicians India. 2006 Mar;54:219-34. J Assoc Physicians India. 2006. PMID: 16800350
-
[Effectiveness and problems of PZA-containing 6-month regimen for the treatment of new pulmonary tuberculosis patients].Kekkaku. 2001 Jan;76(1):33-43. Kekkaku. 2001. PMID: 11211781 Review. Japanese.
-
Optimizing treatment outcome of first-line anti-tuberculosis drugs: the role of therapeutic drug monitoring.Eur J Clin Pharmacol. 2016 Aug;72(8):905-16. doi: 10.1007/s00228-016-2083-4. Epub 2016 Jun 15. Eur J Clin Pharmacol. 2016. PMID: 27305904 Review.
References
-
- World Health Organization. Global Tuberculosis Report 2017. Geneva, Switzerland: World Health Organization; 2017. pp. 1–262.
-
- Iyawoo K. Tuberculosis in Malaysia: Problems and prospect of treatment and control. Tuberculosis (Edinb) 2004;84:4–7. - PubMed
-
- Dony JF, Ahmad J, Khen Tiong Y. Epidemiology of tuberculosis and leprosy, Sabah, Malaysia. Tuberculosis (Edinb) 2004;84:8–18. - PubMed
-
- Clinical Practice Guidelines on Management of Tuberculosis. 3rd ed. Putrajaya: Malaysia Heath Technology Assessment Section (MaHTAS); 2012. [Last accessed on 2018 Feb 01]. Available from: http://www.moh.gov.my .