Nucleus Accumbens Shell Orexin-1 Receptors Are Critical Mediators of Binge Intake in Excessive-Drinking Individuals
- PMID: 30814925
- PMCID: PMC6381036
- DOI: 10.3389/fnins.2019.00088
Nucleus Accumbens Shell Orexin-1 Receptors Are Critical Mediators of Binge Intake in Excessive-Drinking Individuals
Abstract
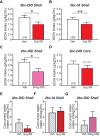
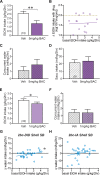
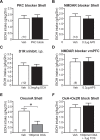
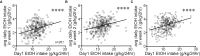
Excessive, binge alcohol drinking is a potent and pernicious obstacle to treating alcohol use disorder (AUD), and heavy-drinking humans are responsible for much of the substantial costs and harms of AUD. Thus, identifying key mechanisms that drive intake in higher-drinking individuals may provide important, translationally useful therapeutic interventions. Orexin-1-receptors (Ox1Rs) promote states of high motivation, and studies with systemic Ox1R inhibition suggest a particular role in individuals with higher intake levels. However, little has been known about circuits where Ox1Rs promote pathological intake, especially excessive alcohol consumption. We previously discovered that binge alcohol drinking requires Ox1Rs in medial nucleus accumbens shell (Shell), using two-bottle-choice Drinking-in-the-Dark (2bc-DID) in adult, male C57BL/6 mice. Here, we show that Shell Ox1Rs promoted intake during intermittent-access alcohol drinking as well as 2bc-DID, and that Shell inhibition with muscimol/baclofen also suppressed 2bc-DID intake. Importantly, with this large data set, we were able to demonstrate that Shell Ox1Rs and overall activity were particularly important for driving alcohol consumption in higher-drinking individuals, with little overall impact in moderate drinkers. Shell inhibition results were compared with control data combined from drug treatments that did not reduce intake, including NMDAR or PKC inhibition in Shell, Ox1R inhibition in accumbens core, and systemic inhibition of dopamine-1 receptors; these were used to understand whether more specific Shell Ox1R contributions in higher drinkers might simply result from intrinsic variability in mouse drinking. Ineffectiveness of Shell inhibition in moderate-drinkers was not due to a floor effect, since systemic baclofen reduced alcohol drinking regardless of basal intake levels, without altering concurrent water intake or saccharin consumption. Finally, alcohol intake in the first exposure predicted consumption levels weeks later, suggesting that intake level may be a stable trait in each individual. Together, our studies indicate that Shell Ox1Rs are critical mediators of binge alcohol intake in higher-drinking individuals, with little net contribution to alcohol drinking in more moderate bingers, and that targeting Ox1Rs may substantially reduce AUD-related harms.
Keywords: SB-334867; alcohol; binge; excessive drinkers; nucleus accumbens; orexin; shell.
Figures

Similar articles
-
Nucleus Accumbens Shell and mPFC but Not Insula Orexin-1 Receptors Promote Excessive Alcohol Drinking.Front Neurosci. 2016 Aug 30;10:400. doi: 10.3389/fnins.2016.00400. eCollection 2016. Front Neurosci. 2016. PMID: 27625592 Free PMC article.
-
Nucleus accumbens shell Orexin-1 receptors are not needed for single-bottle limited daily access alcohol intake in C57BL/6 mice.Alcohol. 2020 Dec;89:139-146. doi: 10.1016/j.alcohol.2020.09.003. Epub 2020 Sep 25. Alcohol. 2020. PMID: 32987129 Free PMC article.
-
Differential importance of nucleus accumbens Ox1Rs and AMPARs for female and male mouse binge alcohol drinking.Sci Rep. 2021 Jan 8;11(1):231. doi: 10.1038/s41598-020-79935-2. Sci Rep. 2021. PMID: 33420199 Free PMC article.
-
Recent Perspectives on Sex Differences in Compulsion-Like and Binge Alcohol Drinking.Int J Mol Sci. 2021 Apr 6;22(7):3788. doi: 10.3390/ijms22073788. Int J Mol Sci. 2021. PMID: 33917517 Free PMC article. Review.
-
Holiday Heart Syndrome: A Literature Review.Cureus. 2025 Feb 28;17(2):e79816. doi: 10.7759/cureus.79816. eCollection 2025 Feb. Cureus. 2025. PMID: 40161046 Free PMC article. Review.
Cited by
-
Neurobiology of the Orexin System and Its Potential Role in the Regulation of Hedonic Tone.Brain Sci. 2022 Jan 24;12(2):150. doi: 10.3390/brainsci12020150. Brain Sci. 2022. PMID: 35203914 Free PMC article. Review.
-
Contribution of hypothalamic orexin (hypocretin) circuits to pathologies of motivation.Br J Pharmacol. 2024 Nov;181(22):4430-4449. doi: 10.1111/bph.17325. Epub 2024 Sep 24. Br J Pharmacol. 2024. PMID: 39317446 Review.
-
The orexin (hypocretin) neuropeptide system is a target for novel therapeutics to treat cocaine use disorder with alcohol coabuse.Neuropharmacology. 2021 Feb 1;183:108359. doi: 10.1016/j.neuropharm.2020.108359. Epub 2020 Oct 19. Neuropharmacology. 2021. PMID: 33091458 Free PMC article. Review.
-
Novel Agents for the Pharmacological Treatment of Alcohol Use Disorder.Drugs. 2022 Feb;82(3):251-274. doi: 10.1007/s40265-021-01670-3. Epub 2022 Feb 8. Drugs. 2022. PMID: 35133639 Free PMC article. Review.
-
The role of beta- and alpha-adrenergic receptors on alcohol drinking.Neuropharmacology. 2023 Aug 15;234:109545. doi: 10.1016/j.neuropharm.2023.109545. Epub 2023 Apr 25. Neuropharmacology. 2023. PMID: 37100382 Free PMC article.
References
-
- Alcaraz-Iborra M., Navarrete F., Rodriguez-Ortega E., De La Fuente L., Manzanares J., Cubero I. (2017). Different molecular/behavioral endophenotypes in C57BL/6J mice predict the impact of OX1 receptor blockade on binge-like ethanol intake. Front. Behav. Neurosci. 11:186. 10.3389/fnbeh.2017.00186 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

