Extreme Neuroplasticity of Hippocampal CA1 Pyramidal Neurons in Hibernating Mammalian Species
- PMID: 30814935
- PMCID: PMC6381046
- DOI: 10.3389/fnana.2019.00009
Extreme Neuroplasticity of Hippocampal CA1 Pyramidal Neurons in Hibernating Mammalian Species
Abstract
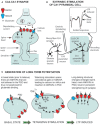
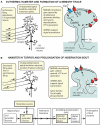
In awake and behaving mammals (with core and brain temperatures at ~37°C), hippocampal neurons have anatomical and physiological properties that support formation of memories. However, studies of hibernating mammalian species suggest that as hippocampal temperature falls to values below ~10°C, CA1 neurons lose their ability to generate long term potentiation (LTP), a basic form of neuroplasticity. That is, the persistent increase in CA3-CA1 synaptic strength following high-frequency stimulation of CA3 fibers (the hallmark of LTP generation at 37°C) is no longer observed at low brain temperatures although the neurons retain their ability to generate action potentials. In this review, we examine the relationship of LTP to recently observed CA1 structural changes in pyramidal neurons during the hibernation cycle, including the reversible formation of hyperphosphorylated tau. While CA1 neurons appear to be stripped of their ability to generate LTP at low temperatures, their ability to still generate action potentials is consistent with the longstanding proposal that they have projections to neural circuits controlling arousal state throughout the hibernation cycle. Recent anatomical studies significantly refine and extend previous studies of cellular plasticity and arousal state and suggest experiments that further delineate the mechanisms underlying the extreme plasticity of these CA1 neurons.
Keywords: LTP; hibernation; hippocampus; memory; neuroplasticity; pyramidal cells (PC).
Figures
Similar articles
-
Decreasing temperature shifts hippocampal function from memory formation to modulation of hibernation bout duration in Syrian hamsters.Am J Physiol Regul Integr Comp Physiol. 2011 Aug;301(2):R438-47. doi: 10.1152/ajpregu.00016.2011. Epub 2011 May 11. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21562095
-
Syrian hamster neuroplasticity mechanisms fail as temperature declines to 15 °C, but histaminergic neuromodulation persists.J Comp Physiol B. 2017 Jul;187(5-6):779-791. doi: 10.1007/s00360-017-1078-5. Epub 2017 Apr 9. J Comp Physiol B. 2017. PMID: 28391591
-
A computational study on plasticity during theta cycles at Schaffer collateral synapses on CA1 pyramidal cells in the hippocampus.Hippocampus. 2015 Feb;25(2):208-18. doi: 10.1002/hipo.22365. Epub 2014 Sep 25. Hippocampus. 2015. PMID: 25220633
-
Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information.Hippocampus. 2001;11(5):578-98. doi: 10.1002/hipo.1073. Hippocampus. 2001. PMID: 11732710 Review.
-
Voltage Imaging in the Study of Hippocampal Circuit Function and Plasticity.Adv Exp Med Biol. 2015;859:197-211. doi: 10.1007/978-3-319-17641-3_8. Adv Exp Med Biol. 2015. PMID: 26238054 Review.
Cited by
-
Biofluid-based biomarkers for Alzheimer's disease-related pathologies: An update and synthesis of the literature.Alzheimers Dement. 2022 Sep;18(9):1687-1693. doi: 10.1002/alz.12618. Epub 2022 Feb 25. Alzheimers Dement. 2022. PMID: 35213777 Free PMC article. Review.
-
TrkB signaling regulates the cold-shock protein RBM3-mediated neuroprotection.Life Sci Alliance. 2021 Feb 9;4(4):e202000884. doi: 10.26508/lsa.202000884. Print 2021 Apr. Life Sci Alliance. 2021. PMID: 33563652 Free PMC article.
-
Neuronal Activity in the Hibernating Brain.Front Neuroanat. 2019 Jul 9;13:71. doi: 10.3389/fnana.2019.00071. eCollection 2019. Front Neuroanat. 2019. PMID: 31338028 Free PMC article.
-
Daily Torpor and Sleep in a Non-human Primate, the Gray Mouse Lemur (Microcebus murinus).Front Neuroanat. 2019 Sep 24;13:87. doi: 10.3389/fnana.2019.00087. eCollection 2019. Front Neuroanat. 2019. PMID: 31616258 Free PMC article.
-
Linking Hypothermia and Altered Metabolism with TrkB Activation.ACS Chem Neurosci. 2023 Sep 6;14(17):3212-3225. doi: 10.1021/acschemneuro.3c00350. Epub 2023 Aug 8. ACS Chem Neurosci. 2023. PMID: 37551888 Free PMC article.
References
-
- Arendt T., Stieler J., Strijkstra A. M., Hut R. A., Rüdiger J., Van der Zee E. A., et al. . (2003). Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J. Neurosci. 23, 6972–6981. 10.1523/JNEUROSCI.23-18-06972.2003 - DOI - PMC - PubMed
-
- Beckman A. L., Stanton T. L. (1982). Properties of the CNS during the state of hibernation, in Neural Basis of Behavior, ed Beckman A. (Jamaica, NY: Spectrum Publications; ), 19–45.
-
- Bhowmick S., Moore J. T., Kirschner D. L., Drew K. L. (2017). Arctic ground squirrel hippocampus tolerates oxygen glucose deprivation independent of hibernation season even when not hibernating and after ATP depletion, acidosis, and glutamate efflux. J. Neurochem. 142, 160–170. 10.1111/jnc.13996 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

