Acinetobacter baumannii Ventilator-Associated Pneumonia: Clinical Efficacy of Combined Antimicrobial Therapy and in vitro Drug Sensitivity Test Results
- PMID: 30814950
- PMCID: PMC6381041
- DOI: 10.3389/fphar.2019.00092
Acinetobacter baumannii Ventilator-Associated Pneumonia: Clinical Efficacy of Combined Antimicrobial Therapy and in vitro Drug Sensitivity Test Results
Abstract
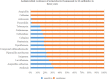
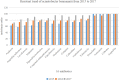
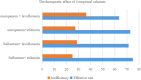
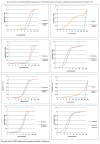
Objective: To evaluate therapeutic efficacy of different combined antimicrobial treatments against Acinetobacter baumannii ventilator-associated pneumonia (VAP). Methods: Clinical outcomes were retrospectively analyzed to elucidate the efficacy of four combined antimicrobial regimens. The chessboard and micro broth dilution methods determined the minimum inhibitory concentrations (MICs) of four antiseptic drugs singly used and combined two drugs against 36 isolates of multidrug-resistant (MDR) A. baumannii. Results: The incidence of VAP was approximately 6.9% (237/3424) between January 1, 2015 and December 31, and 35.9% (85/237) of the cases were caused by A. baumannii. Among these cases, 60 belonged to AB-VAP, for whom antimicrobial treatment plan was centralized and clinical data was complete. Moreover, all 60 strains of A. baumannii were MDR bacteria from reports microbiological laboratory. Resistance rate was lowest for amikacin (68.3%) and ampicillin sulbactam (71.7%). Resistance rate for imipenem increased from 63.2 to 90.9% during the 3 years. However, in these 60 cases of AB-VAP, the combination between 4 antibiotics was effective in most cases: the effective rate was 75% (18/24) for sulbactam combined with etilmicin, 71.4% (10/14) for sulbactam combined with levofloxacin, 72.7% (8/11) for meropenem combined with etilmicin, and 63.6% (7/11) for meropenem combined with levofloxacin. There was no statistical difference between four regimens (P > 0.05). Sulbactam combined with etilmicin decreased 1/2 of MIC50 and MIC90 of sulbactam while the decreases in etilmicin were more obviously than single drug. When adopting meropenem combined with levofloxacin or etilmicin, the MIC of meropenem reduced to 1/2 of that in applying single drug. As for sulbactam or meropenem combined with levofloxacin, it also lessened the MIC50 of levofloxacin to 1/2 of that for single drug. FIC results suggested that the effects of four combined antimicrobial regimens were additive or unrelated. When sulbactam was combined with etimicin, the additive effect was 63.89%. Conclusion: Drug combination sensitivity test in vitro may be helpful for choosing antimicrobial treatment plans. Sulbactam or meropenem as the basis of treatment regimens can function as the alternatives against AB-VAP. Sulbactam combined with etimicin has been regarded as a recommended regimen in Suizhou, Hubei, China.
Keywords: Acinetobacter baumannii; combined antimicrobial therapy; in vitro drug sensitivity test; multidrug-resistant; ventilator-associated pneumonia.
Figures
References
-
- Awad L. S., Abdallah D. I., Mugharbil A. M., Jisr T. H., Droubi N. S., El-Rajab N. A., et al. (2017). An antibiotic stewardship exercise in the ICU: building a treatment algorithm for the management of ventilator-associated pneumonia based on local epidemiology and the 2016 Infectious iseases Society of America/American Thoracic Society guidelines. Infect. Drug Resist. 22 17–28. 10.2147/IDR.S145827 - DOI - PMC - PubMed
-
- Ayraud-Thévenot S., Huart C., Mimoz O., Taouqi M., Laland C., Bousseau A., et al. (2012). Control of multi-drug-resistant Acinetobacter baumannii outbreaks in an intensive care unit: feasibility and economic impact of rapid unit closure. J. Hosp. Infect. 82 290–292. 10.1016/j.jhin.2012.08.016 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

