Functional biomimetic nanoparticles for drug delivery and theranostic applications in cancer treatment
- PMID: 30815042
- PMCID: PMC6383616
- DOI: 10.1080/14686996.2018.1528850
Functional biomimetic nanoparticles for drug delivery and theranostic applications in cancer treatment
Erratum in
-
Correction.Sci Technol Adv Mater. 2019 Feb 18;20(1):96. doi: 10.1080/14686996.2019.1574414. eCollection 2019. Sci Technol Adv Mater. 2019. PMID: 30816357 Free PMC article.
Abstract
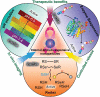
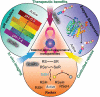
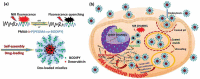
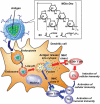
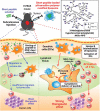
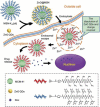
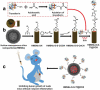
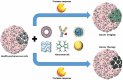
Nanotechnology has been extensively utilized in the design and development of powerful strategies for drug delivery and cancer theranostic. Nanoplatforms as a drug delivery system have many advantages such as in vivo imaging, combined drug delivery, extended circulation time, and systemic controlled release. The functional biomimetic drug delivery could be realized by incorporating stimuli-responsive (pH, temperature, redox potential, etc.) properties into the nanocarrier system, allowing them to bypass biological barriers and arrive at the targeted area. In this review, we discuss the role of internal stimuli-responsive nanocarrier system for imaging and drug delivery in cancer therapy. The development of internal stimuli-responsive nanoparticles is highlighted for precision drug delivery applications, with a particular focus on in vivo imaging, drug release performance, and therapeutic benefits.
Keywords: 10 Engineering and Structural materials; 211 Scaffold / Tissue engineering / Drug delivery; Functional nanoparticles; drug delivery; stimuli-responsive; theranostics.
Figures

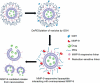
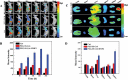
References
-
- Neal JW, Sledge GW.. Decade in review-targeted therapy: successes, toxicities and challenges in solid tumours. Nat Rev Clin Oncol. 2014;11:627–628. - PubMed
-
- Metzger-Filho O, Moulin C, Awada A. Molecular targeted therapy in prevalent tumors: learning from the past and future perspectives. Curr Clin Pharmacol. 2010;5:166–177. - PubMed
-
- DeVita VT Jr., Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68:8643–8653. - PubMed
-
- Cho K, Wang X, Nie S, et al. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–1316. - PubMed
-
- Parveen S, Sahoo SK. Polymeric nanoparticles for cancer therapy. J Drug Target. 2008;16:108–123. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
