Progression of glucose-lowering diabetes therapy in TECOS
- PMID: 30815579
- PMCID: PMC6354756
- DOI: 10.1002/edm2.53
Progression of glucose-lowering diabetes therapy in TECOS
Abstract
Aims: TECOS was a randomized, double-blind, placebo-controlled trial assessing the impact of sitagliptin vs. placebo on cardiovascular outcomes when added to usual care in patients with type 2 diabetes. We report the use of concomitant diabetes medications and the risk for progression to insulin during follow-up.
Materials and methods: TECOS enrolled 14 671 participants with HbA1c 6.5%-8.0% on monotherapy with metformin, pioglitazone, sulfonylurea (SU), or dual therapy with two oral agents or insulin with or without metformin. Subsequent diabetes management was by the participant's usual care physician. Time to initiation of insulin and risk of hypoglycaemia were estimated using Cox proportional hazards models.
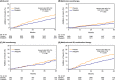
Results: The most common glucose-lowering regimens at baseline were metformin monotherapy (30.2%), SU monotherapy (8.5%), metformin/SU therapy (35.1%), and insulin with or without metformin (13.9% and 8.6%, respectively). Over a median 3.0 years' follow-up, diabetes therapy was intensified in 25.2% of participants (sitagliptin 22.0%, placebo 28.3%). Medications most commonly added were SU (8.3%) or insulin (8.8%). Insulin initiation in the usual care setting occurred at mean (standard deviation) HbA1c of 8.5 (1.5)%. Sitagliptin did not impact rates of severe hypoglycaemia, but delayed progression to insulin when added to metformin or metformin/SU regimens.
Conclusion: Consistent with the trial's pragmatic design, TECOS participants underwent typical progression of diabetes medications. Sitagliptin was associated with lower HbA1c, without increased risk for severe hypoglycaemia and was associated with delayed progression to insulin when added to metformin with or without SU.
Keywords: DPP‐4 inhibitor; hypoglycaemia; insulin; sitagliptin; type 2 diabetes.
Figures


References
-
- Wright A, Burden A, Paisey RB, Cull CA, Holman RR, UK Prospective Diabetes Study Group . Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25:330–336. - PubMed
-
- Swedish National Diabetes Register. https://www.ndr.nu/#/knappen. Accessed March 16, 2018.
-
- Centers for Disease Control and Prevention . Age‐adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011. https://www.cdc.gov/diabetes/statistics/meduse/fig2.htm. Accessed March 16, 2018.
-
- Ng CJ, Lai PS, Lee YK, Azmi SA, Teo CH. Barriers and facilitators to starting insulin in patients with type 2 diabetes: a systematic review. Int J Clin Pract. 2015;69:1050–1070. - PubMed
LinkOut - more resources
Full Text Sources

