Pharmacotherapeutic management of paediatric heart failure and ACE-I use patterns: a European survey
- PMID: 30815586
- PMCID: PMC6361374
- DOI: 10.1136/bmjpo-2018-000365
Pharmacotherapeutic management of paediatric heart failure and ACE-I use patterns: a European survey
Abstract
Objective: To characterise heart failure (HF) maintenance pharmacotherapy for children across Europe and investigate how angiotensin-converting enzyme inhibitors (ACE-I) are used in this setting.
Methods: A Europe-wide web-based survey was conducted between January and May 2015 among European paediatricians dedicated to cardiology.
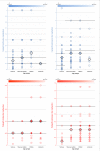
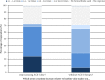
Results: Out of 200-eligible, 100 physicians representing 100 hospitals in 27 European countries participated. All participants reported prescribing ACE-I to treat dilated cardiomyopathy-related HF and 97% in the context of congenital heart defects; 87% for single ventricle physiology. Twenty-six per cent avoid ACE-I in newborns. Captopril was most frequently selected as first-choice for newborns (73%) and infants and toddlers (66%) and enalapril for children (56%) and adolescents (58%). Reported starting and maintenance doses varied widely. Up to 72% of participants follow formal creatinine increase limits for decision-making when up-titrating; however, heterogeneity in the cut-off points selected existed. ACE-I formulations prescribed by 47% of participants are obtained from more than a single source. Regarding symptomatic HF maintenance therapy, 25 different initial drug combinations were reported, although 79% select a regimen that includes ACE-I and diuretic (thiazide and/or loop), 61% ACE-I and aldosterone antagonist; 44% start with beta-blocker, 52% use beta-blockers as an add-on drug. Of the 89 participants that prescribe pharmacotherapy to asymptomatic patients, 40% do not use ACE-I monotherapy or ACE-I-beta-blocker two-drug only combination.
Conclusions: Despite some reluctance to use them in newborns, ACE-I seem key in paediatric HF treatment strategies. Use in single ventricle patients seems frequent, in apparent contradiction with current paediatric evidence. Disparate dosage criteria and potential formulation-induced variability suggest significant differences may exist in the risk-benefit profile children are exposed to. No uniformity seems to exist in the drug regimens in use. The information collected provides relevant insight into real-life clinical practice and may facilitate research to identify the best therapeutic options for HF children.
Keywords: cardiology; paediatric practice; pharmacology; therapeutics.
Conflict of interest statement
Competing interests: All authors report grants from European Union Seventh Framework Programme during the conduct of the study.
Figures




References
-
- Rossano JW, Dipchand AI, Edwards LB, et al. . The registry of the international society for heart and lung transplantation: nineteenth pediatric heart transplantation report—2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplantation 2016;35:1185–95. 10.1016/j.healun.2016.08.018 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
